Uric acid has been related to a tendency to precipitate to form crystals, presenting asymptomatically, until the formation of arthritis, tophi or renal lithiasis. Previously, the presence of asymptomatic hyperuricaemia has been associated with the presence of cardiovascular disease.
ObjectivesTo determine the association of complex coronary artery disease in patients with asymptomatic hyperuricaemia.
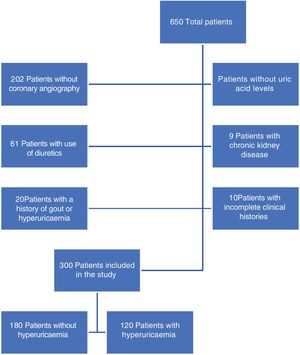
Material and methodsAn observational retrospective, transversal, unicentric study was conducted in a tertiary hospital in Mexico, in the period from June 2017 to March 2019. All patients admitted for coronary angiography were included; patients with gout, use of diuretics and chronic kidney disease were excluded.
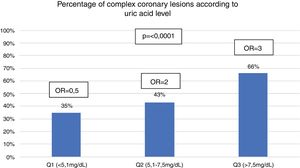
ResultsDuring the study period, a total of 300 patients were collected, of which 40% presented hyperuricaemia. The patients with hyperuricaemia were older (59 vs. 63, P=.002). The group of patients with asymptomatic hyperuricaemia had a higher proportion of complex coronary lesions (64 vs. 35%, P≤.0001) as well as a higher SYNTAX I score (27 vs. 17, P≤.001). There was a higher probability of presenting complex coronary lesions in this group of patients (OR 3.4, P≤.0001). In addition, in the group division of uric acid levels, it was related to the presence of complex coronary lesions (Q1=.5, P=.06), (Q2=2, P=.01) and (Q3=3, P≤.0001).
ConclusionAsymptomatic hyperuricaemia has a higher prevalence and association of presenting complex coronary lesions.
El ácido úrico se ha relacionado con la tendencia de precipitarse para formar cristales, que se presenta desde manera asintomática hasta con artritis, tofos o litiasis renal. Con anterioridad, se ha asociado la hiperuricemia asintomática a la presencia de enfermedad cardiovascular.
ObjetivosDeterminar la asociación de enfermedad arterial coronaria compleja en pacientes con hiperuricemia asintomática.
Material y métodosSe realizó estudio observacional, transversal, retrospectivo, unicéntrico. En un hospital de tercer nivel de México, en el periodo comprendido de junio del 2017 a marzo del 2019. Se incluyó a todos los pacientes que ingresaron para realizar angiografía coronaria; se excluyó a los pacientes con gota, uso de diuréticos y enfermedad renal crónica.
ResultadosDurante el periodo del estudio se seleccionó a un total de 300 pacientes, de los cuales 40% presentaron hiperuricemia. Los pacientes con hiperuricemia eran de mayor edad (59 vs. 63; p=0,002). El grupo de pacientes con hiperuricemia asintomática tuvo mayor proporción de lesiones coronarias complejas (64 vs. 35%; p≤0,0001), así como también mayor puntuación del SYNTAX I score (27 vs. 17; p≤0,001). Hubo mayor probabilidad de presentar lesiones coronarias complejas en este grupo de pacientes (OR 3,4; p≤0,0001). Además, en la división por grupos de nivel de ácido úrico, se relacionaba con la presencia de lesiones coronarias complejas (Q1=0,5; p=0,06); (Q2=2; p=0,01) y (Q3=3; p≤0,0001).
ConclusiónLa hiperuricemia asintomática tiene mayor prevalencia y asociación de presentar lesiones coronarias complejas.
Uric acid is the final product of the metabolism of purine,1 the level of which may be altered by diverse factors, exogenously (ingestion) or endogenously (liver production) or through the participation of renal excretion.2
Historically it has been known that a rise in uric acid levels tends to precipitate the formation of crystals which present asymptomatically until there is clinical evidence such as arthritis, tophi or kidney stones.3 For this reason, the rheumatologist and internal medicine physician have been responsible for diagnosis and treatment although for more than 2 decades its cardiovascular relationship has been studied in greater detail and it has been associated with metabolic syndrome, high blood pressure, ischaemic heart disease, cerebral vascular disease, heart failure and peripheral arterial diseases.2,4–7
It has been proposed that the relationship with cardiovascular disease is due to the fact that xanthine oxidase is an important source of reactive oxygen species, which contributes to inflammation and endothelial dysfunction, atherosclerosis and to coronary slow flow phenomenon.4,8 Also, uric acid stimulates the vascular proliferation of smooth muscle and oxidative stress via the rennin-angiotensin-aldosterone system.9 Furthermore it has been related to certain levels of reactive C protein, interleukin 6, interleukin 18 and tumour necrosis factor alfa.10 Another via which has been related is the promoter of thrombosis and activation of monocyte chemotactic protein-1, which directly impacts the development of atherosclerosis.11
There is scarce literature on the Mexican population regarding the association of asymptomatic hyperuricaemia with cardiovascular disease and even less directly related to coronary lesions.
The aim of this study is to determine the association of asymptomatic hyperuricaemia with complex coronary arterial disease.
Material and methodsA retrospective, observational, single centre cross-sectional study was conducted in the hospital civil de Guadalajara Fray Antonio Alcalde, which is a tertiary hospital situated in the West of Mexico. The period of study was between June 2017 and March 2019.
All patients over 18 years of age who had been admitted to the cardiology service for a coronary angiography and whose serum uric acid levels had been determined, were included. Patients with diagnosis of gout, or with a history of hyperuricaemia, patients in diuretic treatment or with chronic renal disease with glomerular filtration rates <30mL/min/1.73m2 calculated using the MDRD formula were excluded.
Hyperuricaemia was defined as above 7mg/dL in men and above 6mg/dL in women. Dyslipidaemia was defined as the presence of cholesterol >200mg/dL or tryglicerides >150mg/dL. Obesity was defined as a body mass index >30kg/m2. High blood pressure and diabetes mellitus were described by the before-mentioned history of the patient, supported by a medical record. Significant angiographic lesions were determined if there had been a lesion higher than 50% in the trunk or over 70% in the other arteries. The SYNTAX I score was used to determine the complexity of the coronary lesions. For giving the SYNTAX I score, the calculator of the web site www.syntaxscore.com was used.
Means and standard deviation were used for parametric and median quantitative variables with interquartile ranges for quantitative non parametric ranges. Qualitative variables were expressed as percentages. To compare qualitative variables χ2 was used, whilst quantitative variables were compared with the Student's t-test or the Mann–Whitney U test, depending on the normality of the variables. Univariate and multivariate analysis was conducted, using logistic regression to determine the association with complex coronary arterial disease, which was defined as the presence of trivascular coronary arterial disease or left coronary trunk disease. The dependent variable was the complex coronary lesion, the independent ones were hyperuricaemia, diabetes mellitus, high blood pressure, dyslipidaemia, obesity, a tobacco habit and aged over 65 years. The uric acid levels were divided into 3 groups by quartiles to determine the proportion and association using the odd ratio between them and the complex coronary lesions. Statistical analysis was performed with the Med Calc version 15 programme, and statistical significance was determined as P<.05.
The study was conducted according to the statutes of the Declaration of Helsinki. It was approved by the Ethics Committee of our hospital.
ResultsDuring the study period a total of 650 patients were included: 300 were those who met with the inclusion criteria (Fig. 1), of whom 40% presented with hyperuricaemia. On comparing patients with and without hyperuricaemia (Table 1), it was found that patients with hyperuricaemia were older, in both groups the male sex was prevalent and there were no differences in body mass index. Within the associated comorbidities reported there was a higher proportion of patients with high blood pressure in the hyperuricaemia group. Regarding diagnosis on admission, acute ST segment elevation coronary syndrome was the most prevalent in both groups. In the hyperuricaemia group there was a higher percentage of acute non ST segment elevation coronary syndrome patients. In the laboratory result no difference was reported in HbA1c, cholesterol or triglyceride levels.
General characteristics of the population.
| Normal n (%) | Asymptomatic hyperuricaemia n (%) | P | |
|---|---|---|---|
| n | 180 (60) | 120 (40) | |
| Age (SD) | 59 (±11) | 63 (±11) | .002 |
| Sex (male) | 140 (78) | 88 (73) | .5 |
| Body mass index (kg/m2) (DE) | 26.6 (±3.5) | 26.7 (±4) | .8 |
| Blood pressure | 87 (48) | 77 (64) | .01 |
| Diabetes mellitus | 96 (53) | 73 (61) | .2 |
| Dyslipidaemia | 80 (44) | 50 (42) | .7 |
| Obesity | 31 (17) | 18 (15) | .7 |
| Tobacco habit | 116 (64) | 80 (67) | .8 |
| Diagnosis | |||
| SICA CEST | 93 (52) | 53 (44) | .2 |
| SICA SEST | 18 (10) | 27 (22) | .005 |
| Unstable angina | 35 (19) | 20 (16) | .6 |
| Stable angina | 34 (19) | 20 (16) | .7 |
| Laboratory | |||
| Uric acid (mg/dL) (DE) | 5.2 (±1) | 8.3 (±1.6) | <.001 |
| Hba1c (%) (IQR) | 6.2 (5.6–8.5) | 6.7 (5.9–8.3) | .1 |
| Cholesterol (mg/dL) (IQR) | 156 (127–188) | 154 (123–196) | .8 |
| Triglycerides (mg/dL) (IQR) | 130 (97–185) | 134 (96–168) | .8 |
SD: standard deviation; Hba1c: glycosylated haemoglobin; IQR: interquartile range; SICA CEST: acute ST segment elevation coronary syndrome; SICA SEST: acute non ST segment elevation coronary syndrome.
After studying the angiographic lesions (Table 2), it was reported that patients with hyperuicaemia had a higher proportion of complex coronary, left coronary trunk lesions, trivascular disease and anterior, circumflex and right coronary disease. Another relevant finding was that patients with hyperuricaemia had a higher score on the SYNTAX.
Angiographic lesions.
| Normal n (%) | Asymptomatic hyperuricaemia n (%) | P | |
|---|---|---|---|
| Complex coronary lesions | 64 (35) | 77 (64) | <.0001 |
| Left coronary trunk | 21 (12) | 32 (27) | .001 |
| Trivascular disease | 57 (32) | 71 (59) | <.0001 |
| Anterior descendent | 130 (72) | 100 (83) | .04 |
| Circumflex | 91 (51) | 88 (73) | .0001 |
| Right coronary | 110 (61) | 89 (74) | .03 |
| SYNTAX I score (SD) | 17 (±12) | 27 (±14) | <.001 |
SD: standard deviation.
The association of different clinical parameters with the presence of complex coronary lesions (Table 3) were determined using univariate and multivariate analysis. In the univariate analysis it was reported that hyperuricaemia, diabetes mellitus, high blood pressure and an age >65 years were associated with the presence of complex coronary lesions but in the multivariate analysis only hyperuricaemia, and diabetes mellitus were separately associated with complex coronary lesions.
Association of complex coronary lesions.
| Univariate analysis (OR) | Multivariate analysis (OR) | |
|---|---|---|
| Asymptomatic hyperuricaemia | 3.2 (95% CI 2–5.2) P≤.0001 | 3.4 (95% CI 2–5.7) P≤.0001 |
| Diabetes mellitus | 2.4 (95% CI 1.5–3.8) P=.0003 | 2.2 (95% CI 1.3–3.7) P=.001 |
| Blood pressure | 2 (95% CI 1.3–3.2) P=.003 | 1.6 (95% CI .9–2.6) P=.08 |
| Dyslipidaemia | 1.1 (95% CI .7–1.7) P=.8. | |
| Tobacco habit | .8 (95% CI .5–1.3) P=.3 | |
| Obesity | 1.6 (95% CI .9–3) P=.1 | |
| Age >65 years | 1.8 (95% CI 1.1–2.8) P=.01 | 1.4 (95% CI .8–2.3) P=.2 |
OR: odds ratio.
When the study population was divided into 3 groups, the uric acid levels (Q1=uric acid<.5.1mg/dL; Q2=uric acid 5.1–7.5mg/dL and Q3=uric acid>7.5mg/dL) it was found that the proportion of patients with complex coronary lesions increased in keeping with the rising in uric acid levels (Q1 35%; Q2 43% and Q3 66%; P≤.0001). Also, it was found that the probability of presenting with complex coronary lesions also increased as the level of uric acid increased (Q1=.5; 95% CI .3–1; P=.06), (Q2=2; 95% CI 1.1–3.4; P=.01) and (Q3=3; 95% CI 1.8–5; P≤.0001) (Fig. 2).
DiscussionIn the study a prevalence of asymptomatic hyperuricaemia in 40%of the patients who had been admitted with ischaemic heart disease was found, which was higher than that reported by López-Pineda et al.12 who described a prevalence of 34.4% in a study in patients with acute coronary syndrome.
There are other previous studies with computerised tomography which report on coronary lesions. One such study is by Kaya et al.13 who demonstrated in a study of 982 patients that the patients with hyperuricaemia presented with greater severity of coronary lesions. Krishnan et al.,8 in a study with 2498 patients, found that the patients with hyperuricaemia also had a higher prevalence and severity of coronary lesions. They also found that each unit of increased uric acid levels increased by 22% the Agatston score. Hyunwook et al.2 reported similar results in a study with 4188 patients, where they also performed an analysis of subgroups and found that males who were older, were not overweight and had no other concomitant diseases had a higher association with coronary lesions.
The use of coronary angiography in search of coronary lesions has also been reported in the literature. Sai et al.14 demonstrated that in patients under 35 years of age, asymptomatic hyperuricaemia was an independent risk factor for the severity of coronary lesions. They even found that it was only below diabetes mellitus in this patient group. Zhang et al.15 studied 607 premenopausal women and reported that patients with hyperuricaemia suffered from a higher proportion of trivascular coronary disease. Dai et al.16 reported that patients under 45 years of age and with a uric acid level >8mg/dL was a predictor of presenting with trivascular disease. Tasic et al.17 reported that there was a correlation between the level of uric acid and coronary lesions of 2 and 3 blood vessels, but in the multivariate analysis this association was lost.
Our results are similar to those found in the previously described literature. We reported that the patients with asymptomatic hyperuricaemia presented with a higher percentage of complex coronary lesions, and that in each one of the coronary arteries, and also greater severity of coronary lesions through the SYNTAX I. There was also a higher association with the presence of complex coronary lesions, even higher than diabetes mellitus, unlike that reported by Sai et al.14 We also found that the percentage and association with complex coronary lesions increased in keeping with the increase in the level of uric acid.
Apart from studies on coronary arteries, many studies have reported on the presence of hyperuricaemia being associated with greater cardiovascular morbidmortality.18–20 It has even been reported that for each 1mg/dL of elevation above the normal value of uric acid, cardiovascular mortality increases from 12% to 40%.3,10,13,21 In patients with acute ST segment elevation coronary syndrome higher mortality was found in the patients with hyperuricaemia.12,22
Due to the evidence of the association of hyperuricaemia with cardiovascular disease, several prospective and retrospective studies were conducted to see if treatment to patients with asymptomatic hyperuricaemia reduced the rate of cardiovascular events, with conflicting results.6,23 It has been described that the use of alopurinol could be associated with the reduction of cardiovascular events.24,25
Results found in the study are encouraging for further prospective studies where specifically the treatment of asymptomatic hyperuricaemia in progression of coronary lesions is sought, to assess whether the possibility exists to stabilise or even reduce lesions found in the coronary arteries.
One of the limitations of this study is that it was a single centre study, with a small sample size and retrospective. Among its strengths is the fact that few studies conducted in Latin America have demonstrated the association of asymptomatic hyperuricaemia with coronary arterial disease. The results found will force us always to monitor the level of uric acid, especially in patients with pre-existing heart conditions and in patients who are carriers of other concomitant diseases. Further clinical trials would be useful where the relationship of treating patients with asymptomatic hyperuricaemia to reduce rates of cardiovascular events is sought.
ConclusionPatients with asymptomatic hyperuricaemia are at greater risk of presenting with complex coronary lesions.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Miranda-Aquino T, Pérez-Topete SE, González-Padilla C, Hernández-del Río JE, Lomelí-Sánchez ÓS, Esturau-Santaló RM, et al. Hiperuricemia asintomática y enfermedad arterial coronaria. Reumatol Clin. 2021;17:263–267.