To update the study of the association between obesity and treatment response in psoriatic arthritis.
MethodsUpdating a systematic review of clinical trials, prospective or retrospective longitudinal studies and case–control studies in psoriatic arthritis in which obesity was assessed as a predictor of efficacy or toxicity. Risks of bias were assessed with validated scales. A meta-analysis of the results of studies with similar outcome variables and weight measurements was performed.
ResultsTwenty-one studies were included (6 review of clinical trials, 6 longitudinal studies, 7 registers and one case–control studie), with moderate quality. The risk of achieving an ACR20 response if weight≥100kg was estimated at OR=1.42 (1–2.08) and that of withdrawing treatment in an OR of 1.60 (95% CI: 1.34–1.92).
ConclusionsThere seems to be a greater risk of withdrawal of treatment due to inefficacy and difficulty in achieving remission in patients with psoriatic arthritis if they are obese.
Actualizar el estudio de la asociación entre obesidad y respuesta al tratamiento en artritis psoriásica.
MétodosActualización de una revisión sistemática previa, incluyendo ensayos clínicos aleatorizados, estudios longitudinales y casos-control en artritis psoriásica en los que se evaluase obesidad como predictor de eficacia o toxicidad. Los riesgos de sesgos se evaluaron con escalas validadas. Se realizó metaanálisis de los resultados de estudios con variables de desenlace y medidas del peso similares.
ResultadosSe incluyeron 21 estudios (6 ensayos clínicos aleatorizados, 6 estudios longitudinales, 7 registros y un caso-control), de calidad en general moderada. El riesgo de no respuesta ACR20 si el peso es≥100kg se estimó en OR=1.42 (1-2.08) y el de retirar el tratamiento en una OR de 1.60 (IC 95%: 1.34-1.92).
ConclusionesParece existir un mayor riesgo de retirada del tratamiento por ineficacia y dificultad para conseguir remisión en pacientes con artritis psoriásica si son obesos.
Over half of patients with psoriatic arthritis (PA) present with at least one associated comorbidity, with its subsequent negative impact on the disease and quality of life.1,2 Recognition and treatment of comorbidities is essential for treating patients with PA safely and effectively, since it is these comorbidities which often have implications on not just function and quality of life, but also on therapeutic decisions.3–6 The combination of comorbidities often hinders treatment of the baseline disease and leads to a lower survival.1,7,8
Obesity and metabolic syndrome are the most outstanding of comorbidities in PA, the complications of which may be diabetes, fatty liver, high blood pressure and cardiovascular events.9,10 The association between obesity and inflammation has been well studied and is due to the fact that the fatty tissue produces proinflammatory factors, the adipokines, which may impact the development and severity of the inflammatory disease.11,12 According to Bhole et al. obesity percentages in patients with PA (n=644), psoriasis (n=448), rheumatoid arthritis (n=350) and the general population are 37%, 29%, 27% and 18% respectively.13 Also, treatment with tumour necrosis factor-alpha inhibitors (TNFα) has been associated with increased weight, in both patients with psoriasis and with PA,14 and this needs to be taken into account for the follow-up of these patients.
In one previous review we described that obesity and the metabolic syndrome could have had a negative effect on PA activity, both in terms of absence of remission and in the impossibility of achieving a minimum drug activity or survival.15 We also identified a study, conducted by Schmajuk et al.,16 which attempted to respond to the question of whether obesity was associated with higher hepatic toxicity by methotrexate. However, this previous review was only based on 7 highly variable studies, and especially regarding response, without confirmation as to whether obesity could be associated with other types of toxicity. Since this review several studies have been published which could help to provide a better understanding of this problem. The aim of this systematic review is therefore to update the review made in 2015 and study the association between obesity and toxicity or the failure to respond in patients with PA.
MethodsThe previous review search strategy was repeated, from the Medline (via PubMed) and Embase databases up until May 2018, with posterior extension of the search to add new terms. For construction of the question the PICO (patient, intervention, comparison and outcome) strategy was followed (P: psoriatic arthritis and obesity; I: indication of drugs currently approved for PA; O: specific or general toxicity, survival, remission or other response criteria indicated specifically in each study). The search strategies used are available as additional material (Appendix B table of supplementary material and strategies)
The following selection criteria of the studies were established: (1) all the patients had to have PA or a differentiated analysis of patients with PA had to exist; (2) the effect of the obesity factor or, failing this, the body mass index (BMI) should have been studied as a main or secondary predictive factor; (3) the outcome (main variable) had to be toxicity either specific or general: hepatotoxitiy (increase in enzymes, fibrosis, cirrhosis, hepatocarcinoma from non-alcoholic fatty liver due to metabolic syndrome), resistance to insulin, dyslipidaemia, high blood pressure, hyperuricaemia, or response to treatment, in terms of outcome validated in PA (survival or retention of drug, remission, minimal disease activity (MDA), response criteria, reduction in scales or function) and (4) the study had to be a clinical trial, a prospective or retrospective longitudinal study or a control case, but case series or cross-over studies were not accepted. Studies where only skin response was measured were excluded.
All figures resulting from the searches were recorded in the programme EndNote® to aid processing. A reviewer (TO) performed the screening by title and abstract and carried out a detailed review of those where doubts existed regarding whether they met or did not meet selection criteria. If doubt ensued, the reviewer consulted other reviewers.
From the articles reviewed in detail, all data was collected from the description of the sample and the aim of the study, its design and duration of follow-up, the drugs used, the definition of obesity and the variables used and their measurement. For the excluded studies, following detailed review, the reason for exclusion was reported.
For assessment of study bias risks the scale suggested by Cochrane was used for the clinical trials17 and the Newcastle-Ottawa scale for observational studies.18
Qualitative analysis of the information collected by study type, population studied, quality and specific results obtained was made. A meta-analysis was also performed with randomised effect models. Due to the variability of the efficacy measures presented, results were extracted as absolute values and were calculated in odds ratio (OR) and confidence intervals (CI). The diversity of calculation was made with I2 statistical tests. To explain diversity sensitivity analyses was performed whenever necessary.
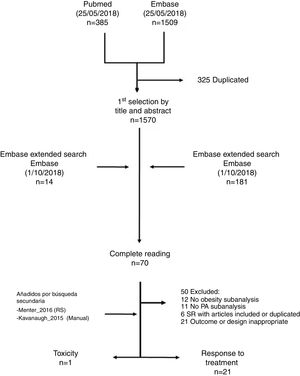
ResultsThe search strategy identified 1894 titles (Fig. 1). Most articles were highlighted by title and abstract, with 70 remaining for complete reading. After reading in detail 2 more studies were added manually (one included in one of the systematic reviews obtained by the strategy19 and the other through knowledge obtained by one of the authors20). Finally, 22 studies were included, of which 21 are described in the table of evidence (Table 1) and the table of results (Table 2). One of the references which resulted from the search was an abstract,21 which was replaced by another indexed reference which had not been found in the initial search.22 The only study found which assessed toxicity was excluded from the description because it had already been included in a previous review.15,16 Furthermore, one of the articles previously included in the review of 2016, that of Cassano et al.23 was rejected in this updating process because it did not measure the outcome in terms of PA activity, only in terms of psoriasis. The studies excluded and their reasons for exclusion were available in a table in the additional material (Appendix B table of supplementary material-excluded).
Table of evidence. Description of included studies which analyse the association of obesity with response.
| Study | Design and duration | na | Drug | Weight/obesity variable | PA response measurement |
|---|---|---|---|---|---|
| Kavanaugh et al., 201520 | RCTc100 weeks | 598 | UST | ≤100kg>100kg | ACR20/50/70 |
| McInnes et al., 201724b | RCTd16 weeks | 422 | ABA | Normal weight: BMI<25Overweight: BMI 25–30Obesity: BMI>30 | Response ACR20 |
| Mease et al., 201525b | RCTe12 weeks | 309 | ADA | Comparison between quartiles (kg)Q1→45.4–73Q2→73.0–84.4Q3→85.0–96.2Q4→97–156 | ΔCDAIΔPsARC |
| Ritchlin et al., 201426 | RCTg1 year | 312 | UST | ≤100kg>100kg | ACR20 |
| Schett et al., 201427b | RCTfAggregated data24 weeks | 1493 | APR | BMI<25BMI 25≤30BMI 30≤35BMI 35≤40BMI≥40 | ACR20ΔHAQ |
| Strober et al., 200828 | RCT24 weeks | 151 | ETN | Normal weight: BMI 18.5–24.9overweight: BMI 25–29.9Obesity: BMI≥30 | ΔPCR |
| Costa et al., 201429 | POLS24 months | 330 | Anti-TNFα | BMI | MDA |
| Di Minno et al., 201340 | POLS24 months | 270 | ETNADAINF | Normal weight: BMI<301st grade Obesity: BMI=30–352nd grade Obesity: BMI 35–40 | MDA |
| Eder et al., 201530b | POLS(2003–2012) | 557 | Anti-TNFα | Normal weight: BMI<25Overweight: BMI 25–30Obesity: BMI>30 | MDA |
| Elkayam et al., 201535b | EOLIsrael registry | 131 | ETNADAINF | Normal weight: BMI<25Overweight: BMI 25–30, 30–35Obesity: BMI>35 | Drug survival |
| Greenberg et al., 201136b | EOLCORRONA registry | 392 | ETNADAINF | Obesity: BMI≥30 | Drug survival |
| Haddad et al., 201331b | POLS | 306 | Anti-TNFαDMARDS | Mayor BMI (no definition of obesity) | MDA |
| Højgaard et al., 201637 | EOLDANBIO+ICEBIO registry | 1943 | ADAETNINF | Not obese: BMI<30Obese: BMI≥30 | Response EULARResponse ACR20/50/70Drug survival |
| Huynh et al., 201722b | EOLCORRONA registry | 325 | Anti-TNFα | No definition of Obesity | Time of withdrawal of drug after reaching MDA |
| Mease et al., 201538 | EOLCORRONA registry | 519 | ADAETNINFOthers | BMI | Drug survivalTime to remission |
| Menter et al., 201619 | EOLPSOLAR registry | 539 | INFADAETNUST | Overweight/obesity class I (BMI≥25–35)Obesity class ii and iii (BMI≥35) | Risk of treatment suspension |
| Ogdie et al., 201639b | EOLCORRONA registry(2005–2013) | 725 | ETNADAINFCTZGOL | BMI | CDAI≤2.8at one year |
| Chiricozzi et al., 201633 | ROLS9 years | 199 | ADA | Healthy: BMI 18.5–24.9Overweight: BMI 25–29.9Obesity: BMI≥30 | Drug survival |
| Fornaro et al., 201734b | ROLS12m | 72 | ADAETNCTZINFUSTGOL | Obesity: BMI≥30 | LUNDEX |
| Iannone et al., 201232 | ROLS36m | 182 | ADAETNINF | Normal weight: BMI<25Overweight: BMI 25–30Obesity: BMI>30 | DAS28/DAS28<2.6SDAI/SDAI<3.3HAQResponse EULAR |
| Brown et al., 201841b | – | 93 | – | Normal: BMI≤24.9Overweight: BMI≥25 | MDA |
ABA: abatacept; ADA: adalimumab; APR: apremilast; PA: psoriatic arthritis; CDAI: Clinical Disease Activity Index; CTZ: certolizumab pegol; DAS: Disease Activity Score; RCT: randomised clinical trial; POLS: prospective observational longitudinal study; ROLS: retrospective observational longitudinal study; ETN: etanercept; DMARDS: disease modifying anti-rheumatic drugs; GOL: golimumab; HAQ: Health Assessment Questionnaire; BMI: body mass index; INF: infliximab; MDA: minimal disease activity; PsARC: Psoriatic Arthritis Response Criteria; SDAI: Simple Disease Activity Index; TNF: tumoral necrosis factor; UST: ustekinumab.
Results from individual studies on the association between obesity and efficacy of psoriatic arthritis treatments.
| Authors, year | Measurement of outcome | Results in relation to the association with obesity/BMI |
|---|---|---|
| Kavanaugh et al., 201520 | -ACR20/50/70 | ACR 20• ≤100kg• PBO→UST: 65.7%• UST 45mg: 61.5%• UST 90mg: 63.5%• >100kg• PBO→UST: 53.3%• UST 45mg: 41.9%• UST 90mg: 64.1%ACR 50• ≤100kg• PBO→UST: 37.3%• UST 45mg: 40.7%• UST 90mg: 47.4%• >100kg• PBO→UST: 37.2%• UST 45mg: 32.6%• UST 90mg: 41%ACR 70• ≤100kg• PBO→UST: 21.6%• UST 45mg: 26.7%• UST 90mg: 23.4%• >100kg• PBO→UST: 9.3%• UST 45mg: 18.6%• UST 90mg: 17.9% |
| McInnes et al., 201724 | -ACR20 | ACR20=no significant difference between obese/overweight and non obese patients-Obese+overweight vs. normal weight or low weight• ABA→OR: 1.215 (.437; 3.378), p=.7087 vs. .446 (.162; 1.228), p=.1181• PBO→OR: .554 (.189; 1.624), p=.2811 vs. .460 (.166; 1.271), p=.1343 |
| Mease et al., 201525 | -CDAI-PsARC | ΔCDAI-Q1→−10.89 (−16; −5.78)-Q2→−10.74 (−15.42; −6.06)-Q3→−8.05 (−14.26; −1.83)-Q4→−10.97 (−16.61; −5.34)ΔPsARC-Q1→.3522 (.1451;.5594)-Q2→.4163 (.2082; .6243)-Q3→.3387 (.1350; .5424)-Q4→.2962 (.0870; .5054) |
| Ritchlin et al., 201426 | -ACR20 | ACR20• PBO∘ ≤100kg=23%∘ >100kg=13.3%• UST 45mg∘ ≤100kg=43.2%∘ >100kg=44.8%• UST 90mg∘ ≤100kg=46.6%∘ >100kg=38.7% |
| Schett et al., 201427 | -ACR20-ΔHAQ | PBO vs. APR20 vs. APR30-ACR 20 depending on baseline weight• <70 (n=336): 20.6 vs. 31.5 vs. 36.4%• 70≤85 (n=454): 21.2 vs. 28.7 vs. 34.6%• 85≤100 (n=391): 17.1 vs. 29.3 vs. 37.2%• ≥100 (n=311): 15.6 vs. 40.7 vs. 41.5%-ACR20 depending on base line BMI• <25 (n=339): 21.4 vs. 34.2 vs. 35.3%• 25≤30 (n=496): 15.8 vs. 27.6 vs. 38.8%• 30≤35 (n=351): 22.4 vs. 38.9 vs. 34.5%• 35≤40 (n=198): 15.2 vs. 23.8 vs. 36.2%• ≥40 (n=107): 17.5 vs. 39.5 vs. 44.8%PBO vs. APR 20 vs. APR 30-HAQ depending on baseline weight• <70 (n=324): −.047 vs. −.128 vs. −.168%• 70≤85 (n=434): −.134 vs. −.182 vs. −.239• 85≤100 (n=383): −.053 vs. −.122 vs. −.225• ≥100 (n=297): −.016 vs. −.224 vs. −.202-HAQ depending on baseline BMI• <25 (n=330): −.052 vs. −.164 vs. −.183• 25≤30 (n=474): −.092 vs. −.180 vs. −.237• 30≤35 (n=343): −.095 vs. −.135 vs. −.239• 35≤40 (n=189): −.014 vs. −.108 vs. −.168• ≥40 (n=102): −.031 vs. −.267 vs. −.165 |
| Strober et al., 200828 | -ΔPCR• Week 12∘ BMI normal: PBO=3.1 (.1–10.1) vs. ETN=3.6 (−3.4; 14.9)∘ BMI overweight: PBO=.5 (−7.2–44.9) vs. ETN=2.2 (−.1; 17.0)∘ BMI Obesity: PBO=−.5 (−8.3–3.4) vs. ETN=2.7 (−.7; 47.3)• Week 24∘ BMI normal: PBO=7.1 (.7–13.8) vs. ETN=2.3 (−2.3, 17.9)∘ BMI overweight: PBO=2.6 (−3.3–45.1) vs. ETN=1.3 (−2.6, 15.1)∘ BMI Obesity: PBO=1.6 (−3.4–11.6) vs. ETN=2.5 (−8.7, 49.5)-Coefficient β (baseline BMI)=−.05 (−.07; −.02); p<.001 | |
| Costa et al., 201429 | -MDA | BMI>30kg/m2→OR=1.29 (1.03–1.64); p=.029 |
| Di Minno et al., 201340 | -MDA | - No MDA vs. MDA• Obese=110 (64%) vs. 25 (25.5%); p<.001• 1st class Obesity=84 (48.8%) vs. 16 (16.3%); p<.001• 2nd class Obesity=26 (15.1%) vs. 9 (9.2%); p<.001-Risk of not obtaining MDA (12 months)• HR if Obesity=4.90 (3.04–7.87); p<.001• HR if 1st class obesity→HR=3.98 (1.96–8.06); p<.01• HR if 2nd class obesity→HR=5.40 (3.09–9.43); p<.01-Risk of probability of maintaining MAR at 24 months→HR=2.04 (1.01–3.61); p=.014 |
| Eder et al., 201530 | -MDA during at least one year | BMI 25–30→OR=.64; p=.001BMI>30→OR=.52; p=.0001 |
| Elkayam et al., 201535 | -Drug survival | ↑BMI→lower survival (data not shown) |
| Greenberg et al., 201136 | -Drug survival | -Risk of interruption in BMI>30: HR adjusted=1.52 (1.08–2.1); p=.017-Drug survival 18 months BMI<30 vs. BMI>30: 80 vs. 63%-Fixed does of anti-TNFα has greater risk of suspension than dose adjusted to weight: HR adjusted 1.3 (.9–2.1); p=.140 |
| Haddad et al., 201331 | -MDA | The patients did not achieve MDA>Baseline BMI (31.6 vs. 28.5); p=.02 |
| Højgaard et al., 201637 | -EULAR and ACR response | In obese patients• Good EULAR response→OR=.75 (.50; 1.15)• Good/moderate EULAR response→OR=.47 (.30; .74); p<.05• Response ACR20→OR=.65 (.40; 1.06)• Response ACR50→OR=.71 (.42; 1.18)• Response ACR70→OR=.80 (.43; 1.48)Survival (for all patients)• Univariate analysis: BMI≥30kg/m2→HR=1.37 (1.18; 1.58)• Multivariate analysis: BMI≥30kg/m2→HR=1.64 (1.32; 2.03) |
| Huynh et al., 201722 | -Time until removal of drug after reaching MDA | Overweight or obesity not significantly affected by disease control loss (data not shown) |
| Mease et al., 201538 | -Drug survival-Time until remission | - anti-TNF survival• BMI→HR=1.030; p=.01, Multivariate: HR=1.011; p=.44-Time until remission• BMI→HR=.940; p<.001, Multivariate: HR=.955; p<.001 |
| Menter et al., 201619 | -Risk of treatment suspension | -First line treatment• Overweight/obesity class i (25≤BMI<35 vs.<25)→HR=.599 (.138–2.609); p=.4951• Obesity class ii–iii (BMI≥35 vs. <25)→HR=1.232 (.258–5.885); p=.7938-Second line treatment• Overweight/obesity class i (25≤BMI<35 vs. <25)→HR=1.753 (.645–4.765); p=.2713• Obesity class ii–iii (BMI ≥35 vs. <25)→HR=1.422 (.473–4.279); p=.5306-Third line treatment• Overweight/obesity class i (25≤BMI<35 vs. <25)→HR=1.026 (.326–3.231); p=.9651• Obesity class ii–iii (BMI≥35 vs. <25)→HR=1.003 (.285–3.533); p=.9967 |
| Odgie et al., 201639 | -CDAI≤2.8 | BMI→OR=.95 (.92–.98) |
| Chiricozzi et al., 201633 | -Drug survival | -BMI 25–30 vs. <25→OR=.91 (.50; 1.68); p=.773-BMI>30 vs. <25→OR=.53 (.22; 1.23); p=.136 |
| Fornaro et al., 201734 | -LUNDEX | -Obese vs. Not obese-Year one: 16 vs. 66%-Year two=10.5 vs. 74.9%-Year three=5.9 vs. 81.8% |
| Iannone et al., 201232 | -HAQ-DAS28-SDAI-DAS28<2.6 (%)-SDAI<3.3 (%)-EULAR response | Normal weight vs. overweight vs. ObesityHAQ=.79 (±.9) vs. .47 (±.8) vs. .81 (±.8); p=.06DAS28=3.1 (±1.6) vs. 2.9 (±1.6) vs. 3.2 (±1.5); p=.42SDAI=14.2 (±13) vs. 11.6 (±12) vs. 13.0 (±12); p=.44DAS28<2.6=44 vs. 46 vs. 37%; p=.31SDAI<3.3=21 vs. 38 vs. 21%; p=.07EULAR response=61.5 vs. 63.8 vs. 62.8%; p=.05 |
| Brown et al., 201841 | -MDA | Overweight→OR=2.99; p=.04 |
ABA: abatacept; APR: apremilast; CDAI: Clinical Disease Activity Index; DAS: Disease Activity Score; ETN: etanercept; HAQ: Health Assessment Questionnaire; HR: hazard ratio; BMI: body mass index; MDA: minimal disease activity; OR: odds ratio; PBO: placebo; PsARC: Psoriatic Arthritis Response Criteria; SDAI: Simple Disease Activity Index; TNF: tumoral necrosis factor; UST: ustekinumab.
Of the studies which researched the association of obesity with efficacy, 6 were clinical trials20,24–28 and 14 were observational studies, out of which 3 were prospective longitudinal,29–31 3 retrospective,32–34 7 analysed recording data19,22,35–39 and another was a control case40 (the study of Brown et al.41 not describing the type of design used and could not be clearly identified).
Of the clinical trials analysed, half of them had an appropriate description of the selection and method of randomisation, but almost all had risks of bias in detection and abandonment during follow-up. Of the observational studies included many were studies sent to congresses, and the data involved were therefore highly limited. For a more detailed analysis of biases, the table of additional material should be consulted (Appendix B table of supplementary material-biases).
The population under study in all studies included patients with active PA and different levels of BMI and mostly treated with anti-TNFα, except in 6 studies, where patients treated with ustekinumab,19,20,26,34 abatacept24 and apremilast27 were also included. The obesity definition used was BMI>30kg/m2, except in one of them22 where it was not defined. In some trials a categorisation of >100kg was added. The most highly studied outcome variables were therapeutic efficacy and drug survival time. The most used outcome measurement was the MDA, but the EULAR and ACR response indexes were also used, as was the Health Assessment Questionnaire (HAQ) for outcome on functions.
Except in the study by Huynh et al.,22 where no relationship was found between loss of disease control and being overweight or obese, the other studies which analysed the MDA showed that this was achieved with greater difficulty and with lower duration of remission in the presence of obesity or overweight.
Regarding the studies which assessed drug survival, they all found differences associated with being overweight, except the study by Chiricozzi et al.,33 which did not find any differences in drug survival between obese and non obese individuals (this was a single centre retrospective study the data of which should be interpreted with caution). The studies by Greenberg et al.36 and Højgaard et al.37 determined that obesity was a risk factor for a lower retention rate, but not clearly due to adverse effects.
Two studies assessed response in terms of change in the HAQ. Iannone et al.32 found that the percentage of patients who achieved significant improvement in functional capacity was similar between the different weight categories (44% in the patient group with normal weight, 53% in those overweight and 50% in obese patients). In the study by Schett et al.27 the 30mg dose of apremilast was associated with an improvement in the HAQ-DI at week 16, which was significant compared with placebo, but no specification was made as to where there were differences between groups with different BMI values.
As has already been commented upon, only one study assessed toxicity.16 This study which had already been included and detailed in the previous review, is a retrospective observational study of patients with psoriatic disease where it was observed that the obese patients in treatment with methotrexate had a tendency for a moderate increase in transaminases. For this study it was decided that no additional analysis of the same would be performed and the reader was referred to the results of the previous review.15
Meta-analysisThe results of all the included articles could not be combined due to the variability in the intervention and in the result measurements. Efficacy measures that could be meta-analysed were ACR20 response and treatment retention.
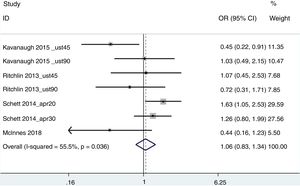
ACR20For this aggregated estimation, the studies of Kavanaugh et al.20 (ustekinumab at a dose of 45 and 90mg), Ritchlin et al.26 (ustekinumab at a dose of 45 and 90mg), Schett et al.27 (apremilast at a dose of 20 and 30mg) and McInnes et al.24 (abatacept 125mg) were included. The patients’ weight was used as a measurement of obesity with 2 groups being established: <100kg and ≥100kg. The result of non response was that of OR=.95 (95% CI .74–1.20), with a diversity of 55.5% (Fig. 2). Eliminating from the analysis the work of Schett et al. (since it was a study with a greater variability, probably because it was an abstract of aggregated data from 3 different clinical trials), the result was OR=1.42 (1–2.08), with an I2 of 6.2%.
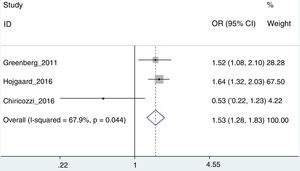
Risk of treatment interruptionOr this analysis, only the works of Greenberg et al.,42 Højgaard et al.37 and Chiricozzi et al.33 could be used, with an aggregated OR of 1.53 (95% CI: 1.28–1.82; I2=67.9%) (Fig. 3). In this case the Chiricozzi et al. Study was the one which offered the greatest diversity (we believe due to several factors, among which was that it was a prospective study). Removal of this study from analysis allowed us to obtain greater homogeneity with an OR of 1.60 (95% CI: 1.34–1.92; I2=0%).
DiscussionDespite the diversity of the studies obtained in this systematic review being high, which complicates the analysis of the whole, there does appear to be a relationship between the risk of treatment failure and obesity in patients with PS. This is so, especially, with the anti-TNF, and at the commonly indicated doses in the prescribing indications for the treatment of PA (which were the most studied), with it being very difficult to extrapolate the results to other molecular targets, although it appears that the effect of ustekinumab20,26 and abatacept,24 at the standard indicated doses from the drug specification sheet could not be modified according to weight. According to the results obtained, the relationship between the drug and obesity appears to be independent both regarding administration route and guidelines followed. The results of the meta-analysis indicate that no differences exist to establish an association between achieving the ACR20 response with ustekinumab or abatacept and weighing over 100kg or under. At this point it is important to highlight that in the drug specification sheet of ustekinumab it establishes that patients who weigh >100kg may use a dose of 90mg43 (endorsed by pharmokinetic stuides44). In the drug specification sheet of golimumab45 it also warns that patients who weigh over 100kg, may increase the dose, if the expected results are not achieved (there are no final data to support this statement).
These findings show the negative impact of obesity in response to the treatment of the patients with PA, making obesity a negative prognostic factor, that may induce a “‘modifying” effect to the treatment which should be taken into account in clinical practice (and treatment prescription) as well as in study design.
Obesity is a low grade chronic state of inflammation through systemic and peregrine increase in levels of cytokines, chemokines and adipokines.46 Apart from having a direct impact on inflammation, obesity may also change the pharmacokinetics of anti-TNF drugs and other biologic agents. Pharmacokinetic studies in anti-TNF agents have identified obesity as a risk factor associated with an increase in drug clearance, which leads to a shorter semilife and in lower concentrations of the drug in serum.47–49 The results of a prospective, observational study were recently made known50 in patients with axial spondyloarthritis, in which the response to the anti-TNF drugs (a response being considered a change in the BASDAI≥2), would be modified in obesity and the use of DMARDS. This could be highly relevant in clinical practice when considering overweight in spondyloarthritis as a significant associated comorbidity.
Only one study16 analysed the relationship between treatment, adverse effects and their relationship with obesity. Schmajuk et al.16 found that obese patients in treatment with methotrexate were at lower risk of raised transaminases, in a probably relationship with baseline fatty liver. It is important to underline the lack of studies that assess the effect of obesity and pharmacological toxicity in patients with PA, only emphasising the relevance of the need to study this relationship and its possible consequences in greater depth. This is of particular interest, given the high prevalence of obesity and its relationshiip with fatty liver, which affects the metabolism of the drugs between patients with PA.13
In the studies which analysed the survival of the drug, we found that withdrawal of the drug was generally made because of its inefficacy,33,36,37,40 rather than the appearance of adverse effects, which reinforces the idea that the obese patient has a high risk of failure to treatment. This was underlined by the results of a meta-analysis, where the obese patients were twice as much at risk of treatment withdrawal.
Other studies which analyse the effect of weight loss (and not directly the effect of obesity or BMI on treatment, which is the aim of this study), propose that weight loss may help to achieve the MDA51,52 and drops in activity, assessed by the DAS28.53 This supports the idea that obesity in PA is a poor prognostic factor and that it plays a major role on the efficacy of different treatments.
It is essential to carry out further research to determine the effect of weight on HAQ, due to the scarcity of data found on the relationship between obesity, response to treatment and its effect on functional capacity.
Similarly, the study tries to assess the effect of being overweight in efficacy and survival of the different molecules in PA treatment. It never attempts to assess the efficacy of these molecules in the treatment of the disease nor compare the different treatments to one another (in the light of the lack of head to head studies, there do not appear to be efficacy differences between them, although abatacept54 and apremilast20,55 appear to be numerically inferior).
This study has certain limitations. On the one hand it focuses on the joints, without considering skin, where a relationship between being overweight and the severity of the disease had already been found.56 There is a physiopathological base that associates lower levels of anti-TNF agents in overweight patients, which could explain the lower drug survival time, at least in the doses usually administered. However, we cannot rule out that this effect could be dose dependent. Another limitation of this study is the fact that studies with different designs were included, which hinders comparison of data. Also, BMI was used to measure obesity but it was not always possible to do this and on some occasions the cut-off point of >100kg in weight was used.
Furthermore, the data found did not asses the ACR50 response, which would be clinically significant, finding changes in the ACR20 response which may not reflect relevant changes in the patients.
In the light of these results, more detailed study of the role of obesity in PA is required, conducting further and better quality studies. However it would be pertinent to bear in mind when prescribing a treatment that obesity may require a specific therapeutic intervention, not only through adjustment of biologic therapy doses, but also directly treating the obesity of these patients, ideally from a multidisciplinary approach.
FinancingMK Media, S.L. financed this review study. No pharmaceutical company was involved in study design or interpretation.
Conflict of interestsT.O. did not receive fees from any pharmaceutical company which could benefit from the results of this review. However, they work for InMusc, which is engaged in methodological consulting for, among other clients, pharmaceutical companies including Abbvie, BMS, MSD, Novartis, Pfizer, Roche, Sarnoff Aventis and UCB Pharma; J.G. has received grants to attend congresses and training activities, fees for scientific consultancy services and fees for conferences and participation in educational programmes for the following companies: Roche, MSD, Pfizer, AbbVie, Janssen Cilag, UCB Pharma, Novartis and Celgene; E.G. states he has received grants to attend congresses and training activities and fees for talks, and educational programmes for Lilly, AbbVie, UCB Pharma, Roche, MSD, Pfizer, Novartis, Celgene and Janssen.
Please cite this article as: Gratacós J, Galíndez E, Otón T. ¿Es la obesidad un factor predictivo de falta de respuesta al tratamiento en la artritis psoriásica? Actualización de una revisión sistemática. Reumatol Clin. 2021;17:268–277.