We report an unusual case of a patient with Behçet's disease that developed protein-losing enteropathy due to intestinal lymphangiectasia.
Se describe el caso de un paciente con enfermedad de Behçet y enteropatía perdedora de proteínas secundaria a linfangiectasia intestinal.
Behçet's disease (BD) is a multi-systemic inflammatory disorder of unknown aetiology with recurrent musculoeskeletal, ocular and mucocutaneous involvement.1 It is worldwide distributed. However, it is more frequent in countries which integrated the old “silk road”.2 Vascular injuries are present in 40% of patients and they may compromise vessels of any size.3 The venous territory is the most frequently affected (75%). Among the most frequent manifestations are thrombophlebitis and thrombosis of lower limbs,4 followed by vena cava thrombosis, pulmonary artery aneurysms and Budd-Chiari syndrome.5,6 The clinical expressiveness of thrombosis varies by virtue of the occlusion degree and the affected anatomical place; a thrombus in the caudal vena cava could be a cause of portal hypertension.
Intestinal lymphangiectasia is a rare but severe cause of protein-losing enteropathy. It is caused by a venous and lymphatic circulation obstruction, which produces an increased blood pressure, with secondary dilation and rupture of lymphatic vessels of the intestinal wall.7 The duodenum is the most affected intestinal portion.8 It manifests as hypoproteinemia, lymphopenia and a decrease of immunoglobulin levels. Elevated levels of alpha-1 antitrypsin in stool confirm the diagnosis.7 It is necessary to perform complementary explorations such as, for example, upper endoscopy or capsule endoscopy, addressed to study the baseline disease such as Menétrier's disease, eosinophilic gastroenteritis and Whipple's disease.9
Below, we present the clinical case of a young man with BD, complicated with portal hypertension by multiple thrombosis, and with protein-losing enteropathy secondary to intestinal lymphangiectasia.
Clinical ObservationA thirty-eight year old man, from Morocco, residing in Spain for 11 years, was diagnosed with BD while he was at 28 years old, resulting from a study of superior vena cava thrombosis and recurrent episodes of folliculitis, pathergy phenomenon, oral and genital ulcers. During the disease progress, he presented thrombosis of the caudal vena cava, right and left branchiocephalic trunk and suprahepatic veins. The patient received a treatment of prednisone 5mg every 24h, azathioprine 50mg every 8h, colchicine 0.5mg every 24h, and aldocumar in movable dosage schedule, although with therapeutic non-compliance due to associated sociopathy.
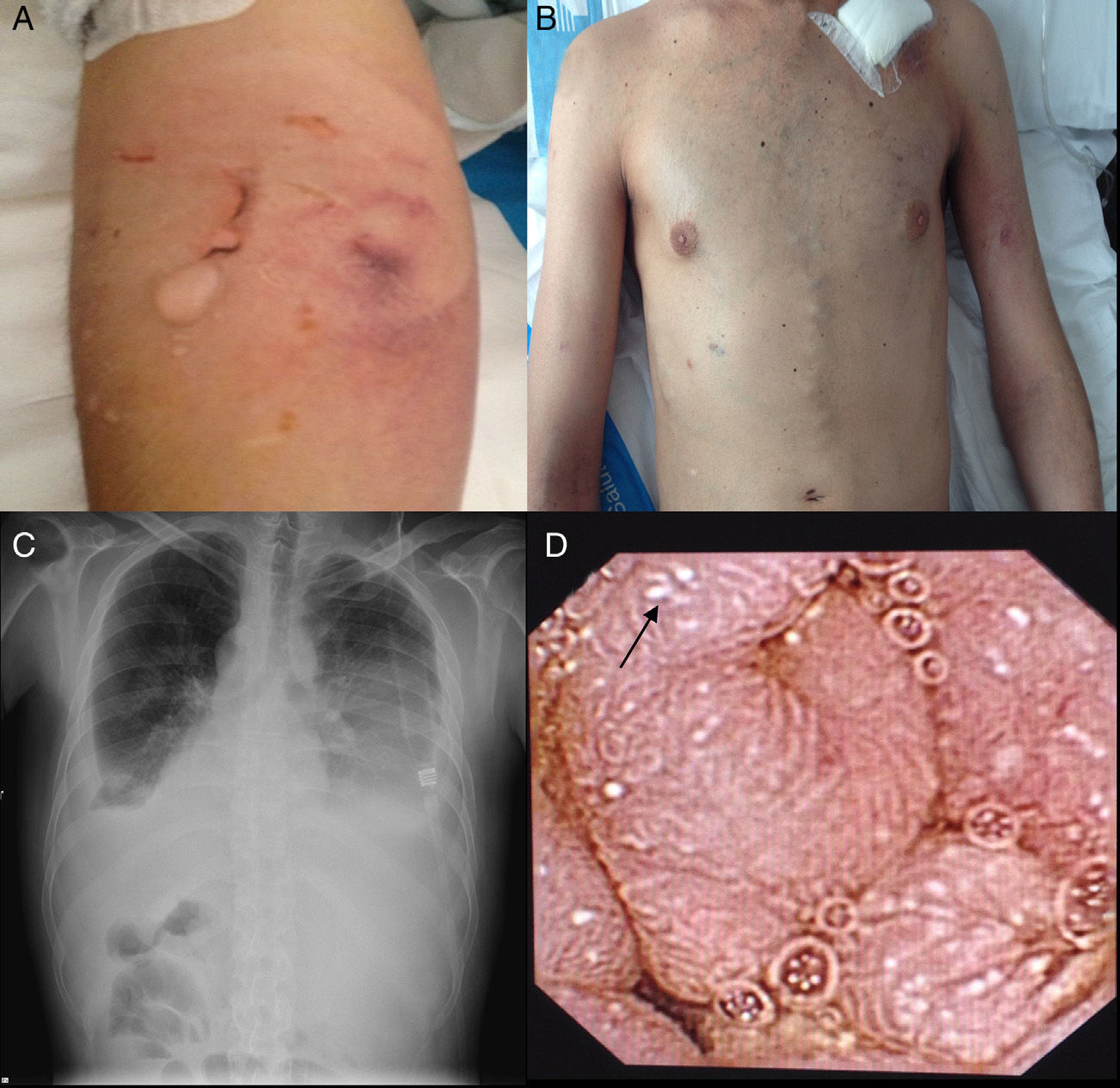
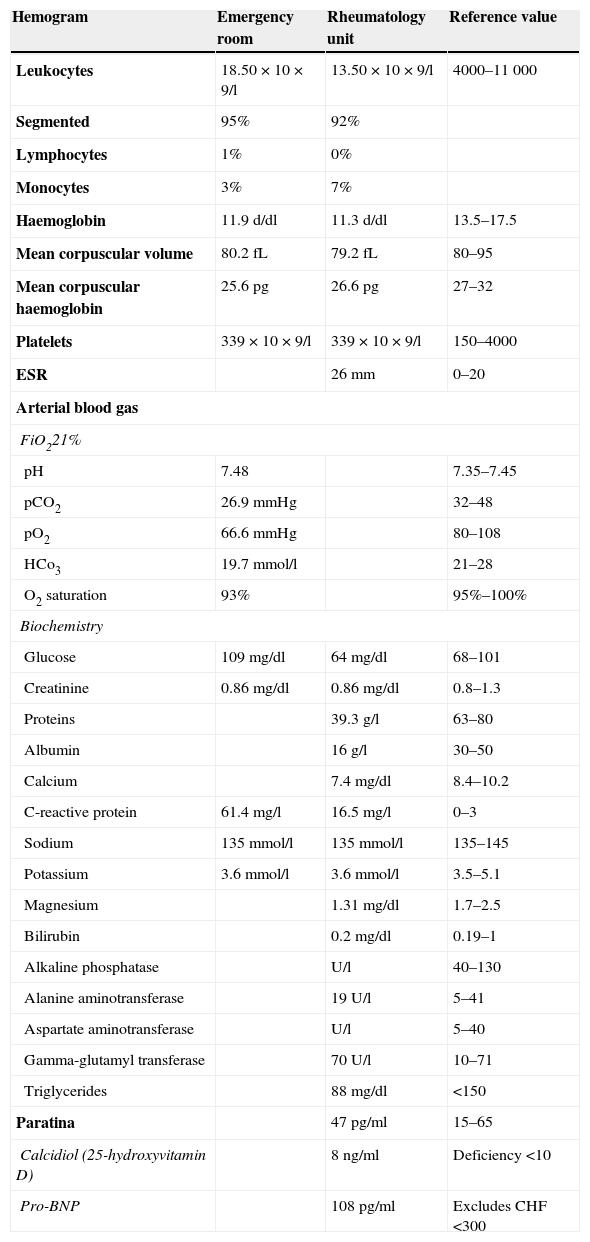
He visited the Emergency Room with dyspnoea, low-grade fever and general discomfort. At the physical exam, there was evidence of marked pallor, tachypnea, 90% baseline oxygen saturation, multiple oral ulcers, folliculitis in arms and supra-pubic area, pathergy phenomenon (Fig. 1A) lower limbs oedema, “caput medusae” collateral circulation in thorax and abdomen (Fig. 1B), hepatomegaly in 4 finger breadths and decreased breath sounds in both lungs at lung auscultation. At the chest X-ray, a bilateral pleural effusion was evidenced (Fig. 1C), for which a thoracentesis was performed, showing an unidentified transudate without presence of germs at Gram staining and negative bacteriological culture. Analytic test showed leukocytosis and elevation of acute phase reactants (Table 1). Upon suspicion of a respiratory infection, empiric antibiotic treatment with meropenem was administered.
Analytical Test Performed in the Emergency Room and Rheumatology Unit.
| Hemogram | Emergency room | Rheumatology unit | Reference value |
|---|---|---|---|
| Leukocytes | 18.50×10×9/l | 13.50×10×9/l | 4000–11000 |
| Segmented | 95% | 92% | |
| Lymphocytes | 1% | 0% | |
| Monocytes | 3% | 7% | |
| Haemoglobin | 11.9d/dl | 11.3d/dl | 13.5–17.5 |
| Mean corpuscular volume | 80.2fL | 79.2fL | 80–95 |
| Mean corpuscular haemoglobin | 25.6pg | 26.6pg | 27–32 |
| Platelets | 339×10×9/l | 339×10×9/l | 150–4000 |
| ESR | 26mm | 0–20 | |
| Arterial blood gas | |||
| FiO221% | |||
| pH | 7.48 | 7.35–7.45 | |
| pCO2 | 26.9mmHg | 32–48 | |
| pO2 | 66.6mmHg | 80–108 | |
| HCo3 | 19.7mmol/l | 21–28 | |
| O2 saturation | 93% | 95%–100% | |
| Biochemistry | |||
| Glucose | 109mg/dl | 64mg/dl | 68–101 |
| Creatinine | 0.86mg/dl | 0.86mg/dl | 0.8–1.3 |
| Proteins | 39.3g/l | 63–80 | |
| Albumin | 16g/l | 30–50 | |
| Calcium | 7.4mg/dl | 8.4–10.2 | |
| C-reactive protein | 61.4mg/l | 16.5mg/l | 0–3 |
| Sodium | 135mmol/l | 135mmol/l | 135–145 |
| Potassium | 3.6mmol/l | 3.6mmol/l | 3.5–5.1 |
| Magnesium | 1.31mg/dl | 1.7–2.5 | |
| Bilirubin | 0.2mg/dl | 0.19–1 | |
| Alkaline phosphatase | U/l | 40–130 | |
| Alanine aminotransferase | 19U/l | 5–41 | |
| Aspartate aminotransferase | U/l | 5–40 | |
| Gamma-glutamyl transferase | 70U/l | 10–71 | |
| Triglycerides | 88mg/dl | <150 | |
| Paratina | 47pg/ml | 15–65 | |
| Calcidiol (25-hydroxyvitamin D) | 8ng/ml | Deficiency <10 | |
| Pro-BNP | 108pg/ml | Excludes CHF <300 | |
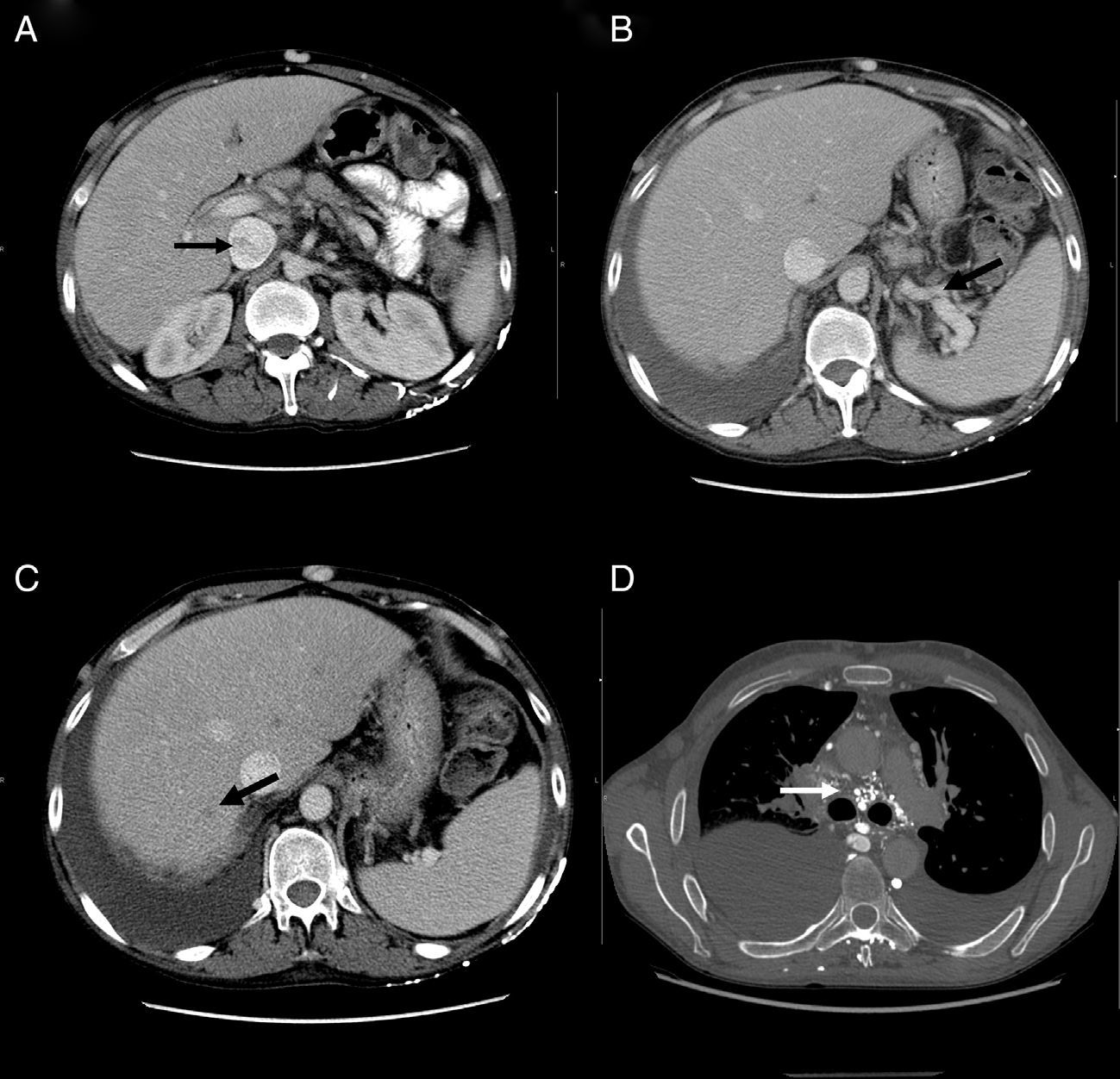
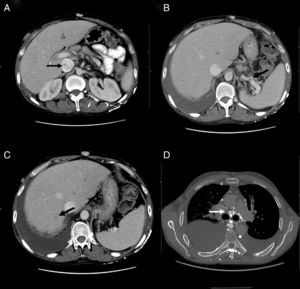
At his admission, increase of lower limbs oedemas was evidenced, in addition to scrotal and abdominal wall oedema. Diuretic treatment was initiated with partial improvements. At admission, the analytical test (Table 1) showed emphasised leukocytes persistence, elevation of acute phase reactants, hypoproteinemia and hypoalbuminemia, and normal hepatic and renal function. Autoimmunity study (anti-nuclear antibodies, extractable nuclear antibodies, anticytoplasmic antibodies, rheumatoid factor, anti-citrulline antibodies), blood cultures, viral serology for hepatitis B and C, and human immunodeficiency virus resulted negative. The echocardiogram showed a minimum pericardial effusion, without signs that suggest constriction. An abdominal Doppler echography confirmed the presence of homogeneous hepatomegaly and mild ascites. No flow in middle and left suprahepatic veins (Budd-Chiari syndrome). Right suprahepatic vein was partially permeable with monophasic flow and the caudal vena cava was slightly enlarged. Also, there was evidence of portal hypertension signs with collateral circulation in the splenic hilum, retroperitoneal and umbilical vein repermeabilisation. These findings have been confirmed with an abdominal tomography (Fig. 2A–C). Urinalysis showed a urine protein of 0.1g/24h. Due to the presence of hypoproteinemia, hypoalbuminemia and vitamin deficit, a malabsorption syndrome was suspected. The results of serum transglutaminase test were also normal. Subcutaneous abdominal fat biopsy was performed and there was no evidence of amyloid material. Colonoscopy and ileoscopy excluded the presence of ileitis. Intestinal lymphangiectasia was suspected due to portal vein hypertension, splanchnic and protein-losing enteropathy, and was confirmed with the elevated excretion of alpha-1 antitrypsin in stool (2.85mg/g reference value <0.3) and compatible images found with capsule endoscopy in the duodenal tract (Fig. 1D). During hospital stay, the patient experienced recurrent episodes of thrombophlebitis, consequently requiring a central line (femoral) inserted at 72h. As it was not possible to administer an intravenous treatment, a central line was inserted using arteriogram, evidencing great collateral supraclavicular circulation and in thorax (Fig. 2D). Internal jugular vein was reduced and external jugular vein was dilated; thrombosis of both innominate trunks with abundant collateral circulation which drained to the pericardiacophrenic vein was observed by means of the contrast injection. Such a vein was catheterised, where a bicameral subcutaneous reservoir was inserted, which the patient could only tolerate for 24h because of pain during drug administration.
Oral treatment was also limited due to intestinal absorption deficiency. The patient received treatment with serum albumin, intravenously furosemide, vitamin supplements and octreotide (peripheral access) with good results. For BD control, metotrexate 15mg weekly was prescribed as well as sodium enoxaparine subcutaneously associated to 10mg of prednisone every 24h; to secure absorption, sublingual administration was chosen.
Moreover, the patient presented episodes of candidiasis and oral herpes which were intravenously treated with antifungal and antiviral agents.
At hospital discharge, there was remission of oedemas and pleural effusion was significantly reduced. Leucocyte and acute phase reactants counts were standardised. His nutritional condition improved considerably.
Currently, after a year since the diagnosis of intestinal lymphangiectasia, the patient is monitored in rheumatologic and digestive external offices.
DiscussionBD is characterised by the presence of recurrent oral-genital ulcers, uveitis, arthritis and skin lesions, and it is the only vasculitis disease that may affect vessels of any size.1 This disease affects mainly veins and presents thrombotic episodes, which in many cases are difficult to manage as they are recurrent and because of the thrombus-related secondary syndromes.6 Intestinal lymphangiectasia is a cause of protein-losing enteropathy, whose physiopatological basis is the intestinal lymphatic vessel dilation. It may be primary or secondary to an obstructive disorder.9 In this case, it is believed that intestinal lymphangiesctasia associated to BD is secondary to portal hypertension caused by multiple thrombi in the portal area. Morita et al.10 evidenced an increase amount of iliopelvic and lumbar lymphatic vessels in lymphogram of patients with BD and intestinal lymphangiectasia; and concluded that dilation of lymphatic channels could be associated to an increased flow due to vascular hyperpermeability and not because of a lymph circulation block.
As illustrated in the present case, due to the low oncotic pressure originated by hypoproteinemia, patients also present ascites, pleural effusion, pericardial effusion and oedemas. This contrasts with the description made by Asakura et al.11 in 1978, where 4 of the 15 patients with intestinal lymphangiectasia did not present serum hypoproteinemia. Because of the coexistence of hypogammaglobulinemia and lymphopenia,6 infections are frequent and may increase mortality.12 However, Tsuchiya et al.13 expressed that, as compared to patients with protein-losing enteropathy, patients with BD and intestinal lymphangiectasia do not present hypogammaglobulinemia.
Diagnosis of lymphangiectasia is made by exclusion, generally in patients with protein-losing enteropathy study, where it is detected as elevated alpha-1-antitrypsin in stool7 and its images are obtained by gastroscopy or capsule endoscopy.14
In this case, as well as in the bibliography, it was not possible to have a histopathological confirmation, as this is performed in a minimum percentage of cases. For example, in a series of 1866 patients with suspect of intestinal lymphangiectasia, histopathological confirmation is performed in only 1.9%.15 Treatment is based on high molecular weight protein replacement, fat restriction, vitamin supplements16,17 and management of the baseline disease. Short chain fat restriction prevents rupture of lymphatic vessels.11 Efficacy of octreotide has been described in the bibliography, despite that its mechanism of action is not clarified.18 Human serum albumin infusion is a supportive treatment which allows maintenance of oncotic pressure and improves oedemas while establishing the background therapy.9 Thrombosis treatment in BD varies according to reviews, without one treatment of preference. The role of anticoagulation in deep venous thrombosis is not properly established and there are dissenting publications.19–21 Retrospective studies show that the anticoagulant treatment is ineffective for the prevention of thrombosis; however, when adding immunosuppressants, the risk factors for rethrombosis significantly decrease. There is little experience with fibrinolytic treatment or stents.22
ConclusionThis case illustrates the complications as regards thrombosis in a patient with BD who develops a secondary intestinal lymphangiectasia. Intestinal lymphangiectasia is to be suspected in patients with BD, portal hypertension and protein-losing enteropathy.
Ethical ResponsibilitiesProtection of persons and animalsAuthors state that no experiments were performed on human beings or animals as part of this investigation.
Data confidentialityAuthors declare that they have complied with the site protocols about the publication of patient data.
Right to privacy and informed consentAuthors have obtained the informed consent from the patients and/or subjects referred to in the article. This document is in possession of the corresponding author.
Conflict of InterestThe authors declare that there are no conflicts of interest.
Please cite this article as: Rodríguez-Muguruza S, Caballero N, Horneros J, Domenech E, Mateo L. Enfermedad de Behçet y enteropatía pierdeproteína secundaria a linfangiectasia intestinal. Reumatol Clin. 2015;11:247–251.