Eosinophilic pneumonia is classified by its acute or chronic presentation, the distinguishing characteristics of which are based on the presence of cough, dyspnea, fever and pulmonary infiltrates with accumulation of inflammatory cells, predominantly eosinophils. The association of eosinophilia and rheumatologic disorders is well known, as in the case of eosinophilic fasciitis and the Churg-Strauss syndrome. The coexistence of chronic eosinophilic pneumonia and rheumatoid arthritis has been reported, either early rheumatoid arthritis of definitive disease. The pathophysiological role of eosinophils in autoimmune diseases is not well defined; however, it has been shown that the production of pro-inflammatory cytokines stimulates and activates different cell groups, and can simultaneously induce autoantibodies and/or increased infiltration of eosinophils in various tissues, without an underlying autoimmune disease. The case of a young woman, with rheumatic chronic eosinophilic pneumonia manifestations and the presence of autoantibodies, which resolved spontaneously, is presented here.
Las neumonía eosinofílica se clasifica por su presentación en aguda o crónica; las características distintivas se basan en la presencia de tos, disnea, fiebre e infiltrados pulmonares con acumulación de células inflamatorias, predominante de eosinófilos. La asociación de eosinofilia y padecimientos reumatológicos es bien conocida, como en el caso de la fascitis eosinofílica y el síndrome de Churg-Strauss. La coexistencia de neumonía eosinofílica crónica y artritis reumatoide ha sido reportada, ya sea de inicio coincidente o en artritis reumatoide establecida. El papel fisiopatológico de los eosinófilos en las enfermedades autoinmunes no está bien definido, sin embargo se ha demostrado que la producción de citocinas proinflamatorias estimulan y activan diferentes grupos celulares, pudiendo en forma simultánea inducir autoanticuerpos e incremento y/o infiltración de eosinófilos en diversos tejidos, sin tener una enfermedad autoinmune subyacente. Presentamos el caso de una mujer joven con neumonía eosinofílica crónica con manifestaciones clínicas reumatológicas y presencia de autoanticuerpos, que se resolvió en forma espontánea.
Eosinophilic pneumonias are a group of diseases extensively studied and described in the literature, which have been classified by their presentation in acute or chronic eosinophilic pneumonia (CEP), and the distinctive features of its presentation are based on the presence of cough, dyspnea, fever and pulmonary infiltrates due to the presence of inflammatory cell accumulation, with predominant eosinophils.1 Acute eosinophilic pneumonia, as its name implies, has a sudden onset, usually associated with moderate to severe hypoxia, which may jeopardize the patients’ life,2 unlike CEP, is a benign form, with an insidious presentation with less respiratory impact.3 In both cases, once the diagnosis is defined, there is a favorable response to glucocorticoids, the basis of its treatment, apparently based on an immunoallergic link underlying diseases such as allergic rhinitis, sinusitis, asthma and atopy.1–3
The association of eosinophilia and rheumatologic diseases has been widely described in literature, and we can highlight as an example and mention some of them as eosinophilic fasciitis syndrome and Churg-Strauss,4,5 but there are other autoimmune diseases such as arthritis own (RA)6 and systemic lupus erythematosus, which described the presence of concomitant phenomena such as eosinophilic vasculitis, panniculitis, enteritis or eosinophillic pneumonia.7 The pathophysiology of this association has not been well elucidated, as the eosinophil is a cell that has been very involved in the phenomena of autoinmunidad.6
We report a woman with symptoms of RA associated with clinical symptoms of chronic eosinophilic pneumonia, in which it is necessary to emphasize the production of autoantibodies and spontaneous remission.
Description of the CaseThe case was a 47-year-old woman, born in the State of Mexico. She had a history of being a smoker since age 16, with a consumption of 3–4 cigarettes a day until age 30 when she stopped. In 1999 she was treated for allergic rhinitis with antihistamines and nasal glucocorticoid drugs and, intermittently, parenteral glucocorticoids (every 6 or 8 months). The patient reported that her illness began early in 2008, with malaise, fever and the presence of joint pain. In later days dyspnea, fatigue and fever were added. She was seen by the family physician, who found the following laboratory results: positive rheumatoid factor (RF) 1:1.280 and eosinophilia of 15% (1605cells/mm3), for which she was referred to our hospital.
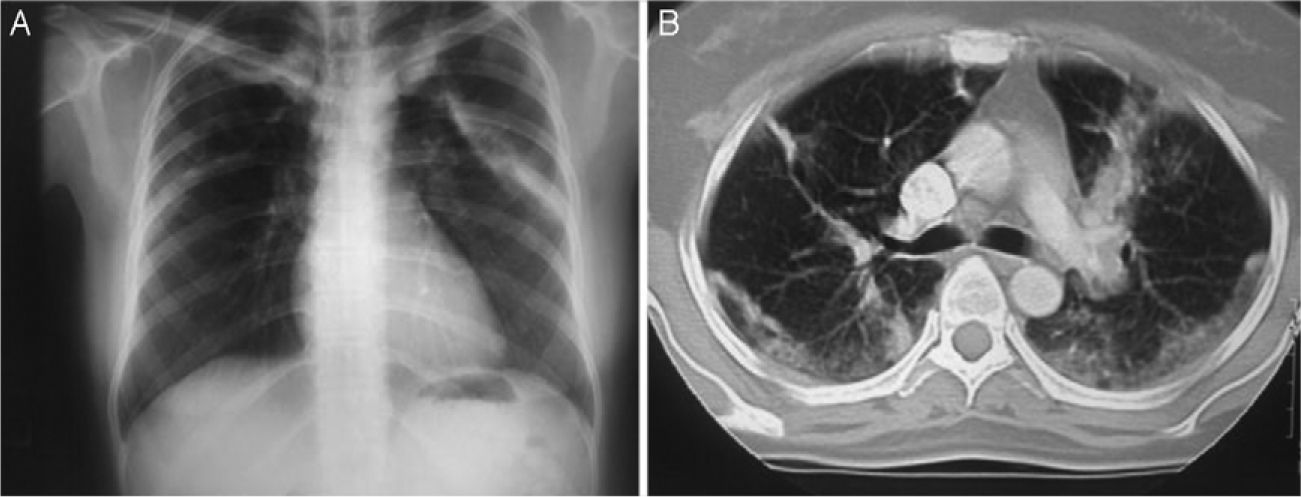
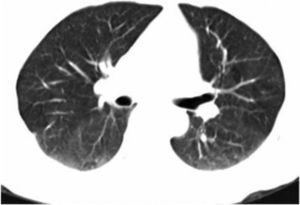
On admission, the patient had rales in the upper third of both lungs, predominantly on the left hemithorax. The musculoskeletal examination showed no inflammatory data or functional limitation. The chest X-ray revealed bilateral pneumonia mainly on the left lung, and computed tomography data found a bilateral pneumonia mainly on the left lung (Fig. 1A and B). The sinus CT scan was normal. Pulmonary function tests were within normal limits, with an oxygen saturation of 95%.
We began a study protocol for eosinophilia, and cytology confirmed eosinophils of 54.3% (4.487cells/mm3), with positive RF by nephelometry 204.5IU/mL, and negative anti-citrullinated peptide antibodies 14.7IU/mL (normal <25), elevated erythrocyte sedimentation rate 81mm/h (Westergren), CRP 40.7<0.8mg/dL, IgE 1596IU/mL (normal <100), c-ANCA 1:80, 1:20 x-ANCA, with ELISA antibodies against proteinase-3 being 0.7U/mL (normal <3.5), and myeloperoxidase 0.7U/mL (normal <9.0), both negative. Human histocompatibility antigen, HLA-DR, was also determined as being DRB1*04. The PPD test was nonreactive. The presence of intestinal parasites was also ruled out by conventional tests.
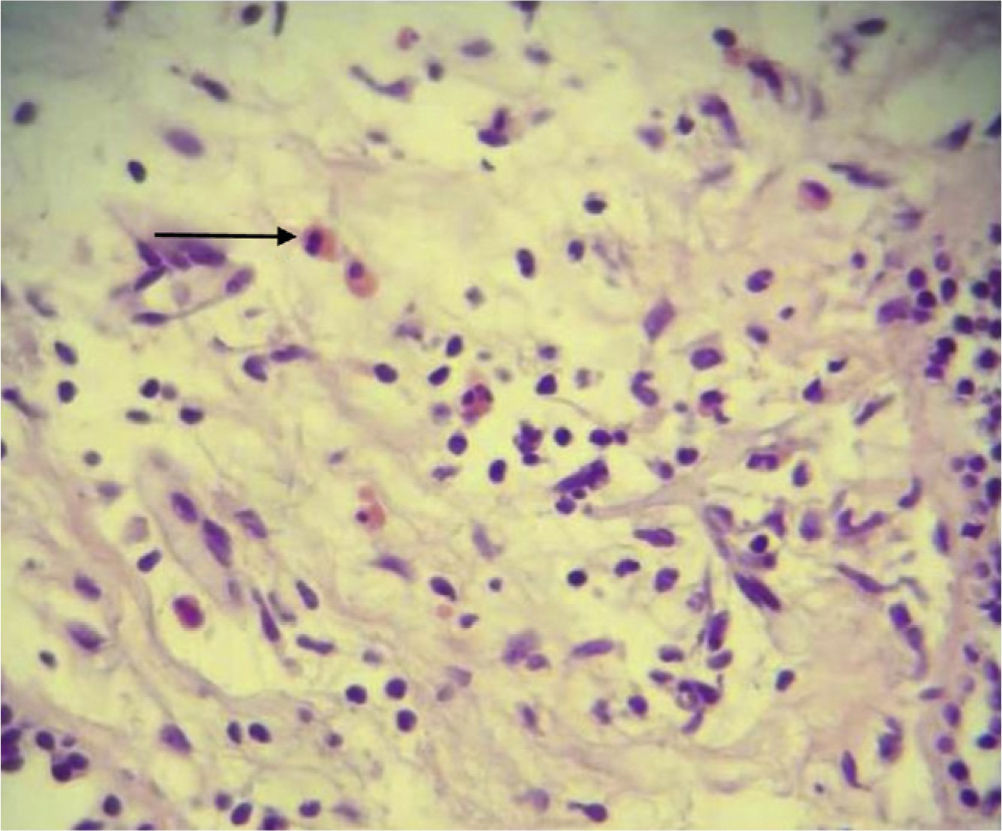
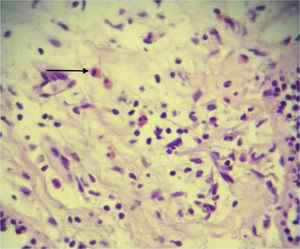
An assessment by the Service of Pneumology suggested a bronchoscopy, and broncho-alveolar lavage (BAL) showed acute and chronic inflammation of the right upper lobe and left upper lobe with non-specific inflammatory reactive changes, where there were lymphocytes, neutrophils and hemosiderin-laden macrophages. Because we failed to establish a definite diagnosis, the patient underwent an endoscopic thoracotomy biopsy of the lingula and posterior left lower lobe, with results compatible with eosinophilic pneumonia (Fig. 2), ruling out the diagnosis of Churg-Strauss syndrome and Wegener's granulomatosis.
The patient's general condition was stable with no evidence of respiratory failure, cough or fever, so it was decided to treat her only with NSAIDs until the follow-up appointment a month later (diclofenac 100mg/day).
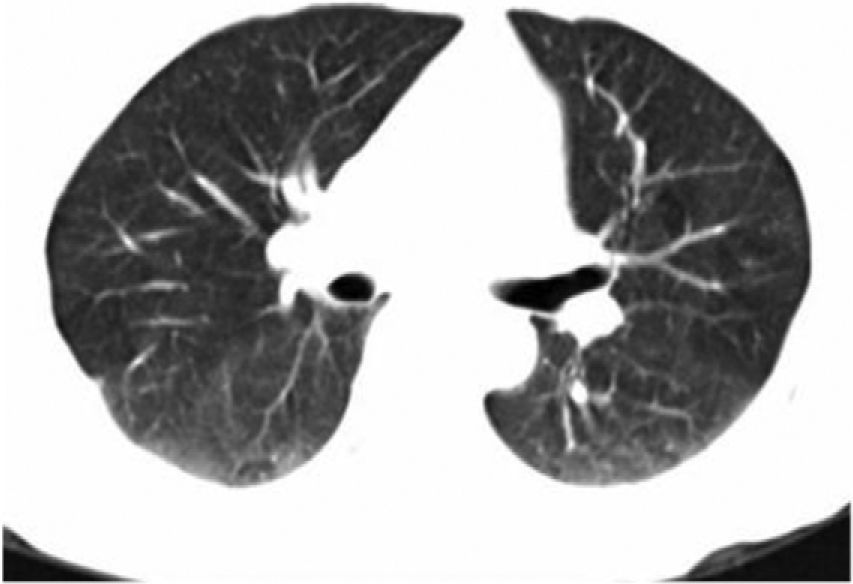
The progression of the patient was satisfactory and after 3 months of follow-up, control studies were performed, showing radiological and tomographic resolution of pneumonia (Fig. 3). Peripheral eosinophilia and immunological studies showed negativity for both the RF 9.13IU/mL (normal <20), and for ANCA, which were undetectable. The patient did not require treatment with corticosteroids or immunosuppressants, and so far, after 3 years of follow-up, the patient remains asymptomatic and serologically negative for autoantibodies.
DiscussionCEP was described by Carrington et al. in 19691 in a patient with a history of rhinitis and asthma, with dyspnea, fever, peripheral eosinophilia and BAL eosinophils. The patient responded favorably to treatment with glucocorticoids. Series and isolated cases point to immunoallergic disorder as a potential cause since, as mentioned previously, most patients have a history of allergies such as asthma, rhinitis and atopy.3,4
The presence of eosinophilia has been described by several authors in association with some autoimmune diseases such as RA, where this phenomenon has been implicated as a response to inflammatory activity.8
Now recognized as being due to the release of cytokines generated in acute inflammatory processes, these proinflammatory cytokines have been identified as interleukin (IL)-6, IL-10, but mainly as IL-5, which, in addition to inducing the production of eosinophilic cells, participates in the inhibition of apoptosis in eosinophils infiltrating the tissue, which explains the greater damage to the target organ.9 A number of other cytokines called chemokines have also been identified, mainly eotaxin, which has a greater power for eosinophil chemotaxis and exotaxin-1 and -2, produced by macrophages, monocytes, fibroblasts and T cells (Th2), which are also a major stimulus in the recruitment of eosinophils, and are responsible for their migration and extended stay at the site of the infiltrated tissue inflammation.9 Interestingly, we have described elevated levels of eotaxin-1 in patients with RA of recent onset upon analyzing role of proinflammatory cytokines present in this pathology. Syversen et al. described this finding, which could correspond to the original description of the association of eosinophilia to RA.10
The presence of eosinophilia is particularly important in some rheumatologic disorders such as Churg-Strauss vasculitis and Wegener's granulomatosis, where its role is crucial in generating the acute inflammatory process in lung11 tissue. Differential diagnosis with eosinophilic granulomatous vasculitis or Churg-Strauss syndrome is complex, as both share similar clinical and serological manifestations, such as the presence of ANCA in up to 40% of cases and RF.12 According to the description of the disease, 3 clinical stages can be recognized. Our case could be in stage 2, having gone through the prodromal phase of allergic disease (rhinitis), and in the second phase show eosinophilic infiltration in different tissues such as the lung (eosinophilic pneumonia), skin and gastrointestinal system.11,12 However, the difference is in the histopathological data showing necrotizing vasculitis and extravascular granulomas, absent in CEP.12
Payne et al. first described the coexistence of CEP and RA in 1980. They reported the case of a 54-year-old male patient of Indian origin with a diagnosis of RA of 17 years, who developed a picture of CEP.13 Other reports have described similar cases with established RA, prior to the presence of clinical symptoms of CEP. Norman et al. described a case with the simultaneous appearance of manifestations of RA and CEP in a 39-year-old whose first manifestation was a dry cough, fever, sweating, fatigue and joint pain, progressing to polyarthritis, establishing the diagnosis of CEP on the basis of clinical criteria and the LBA image but without the presence of peripheral eosinophilia. RF was positive 1:1.280, establishing the diagnosis of RA. Glucocorticoid treatment was administered based on methotrexate and hydroxychloroquine and associated with favorable response, but eventually required treatment with etanercept for control of arthritis. At one year follow-up there was no evidence of pneumonia recurrence.14 A second case of simultaneous presentation, referred by Kwaket al., was in an Asian woman, 55, who started with polyarthritis involving small joints of hands, elevated acute phase reactants and a total eosinophils count of 730/mm3, in addition to positive RF 243IU/mL. She was treated with parenteral glucocorticoids and treatment for the pneumonia, with improvement of the manifestations of arthritis, subsequently adding methotrexate 15mg/week, descending the dose of oral prednisone. Her progression was favorable, with no recurrence of pneumonia after 18 months of follow-up; however, the authors did not comment on whether the presence of RF persisted over time.15
We described the criteria met to establish a clinical and pathological diagnosis of CEP1; however, despite having joint manifestations, we never managed to show objective evidence of acute synovitis, which is essential for the diagnosis of suspected RA, and despite having tested positive for RF and in addition to the DRB1*04 allele, identification of citrullinated peptide antibodies was negative, which together with the absence of arthritis, impeded her classification as RA.16 An important fact is the low titer for c-ANCA autoantigens and x-ANCA, all becoming negative over time as in the case of RF after 3 years of clinical follow-up, persisting undetectable.
We cannot conclude whether there is a link or just a phenomenon of association between patients with established RA and CEP, as in the case of Kwak et al., There is a question of whether over time the patient remained seropositive for RF, or could have had a similar progression to the patient presented here.
A relationship may exist between the two conditions and is probably determined by proinflammatory cytokine production by T cells, which in turn stimulates the production of other cytokines that induce autoantibody production and release eosinophils into the bloodstream, apart from the systemic inflammatory response in the case of RA, and cellular hypersensitivity response in CEP, but we have been unable to clearly establish the link between autoimmune and allergic diseases.
ConclusionsCEP is a clinical condition with an apparently immunoallergic basis, which due to an extensive network of cytokine stimulation and the possible pathogenic role of eosinophils, could be confused with Churg-Strauss syndrome in its eosinophilic clinical phase. The benign course of CEP evidences the difference between the immunoallergic process and autoimmune vasculitis which usually requires chronic treatment with corticosteroids and immunosuppressive agents for control and remission of the disease.
The physician should be aware of the clinical, serological and pathological differences used to establish a correct diagnosis for each of these entities, and thus indicate a specific treatment that allows for the resolution of these pathologies.
Conflict of InterestsThe authors declare no conflicts of interest.
To Dr. Claudia S. Carrillo-Ponce, of the Pathology Department of ISSEMYM, who kindly collaborated in the description of the histological studies.
Please cite this article as: Jaimes-Hernández J, et al. Neumonía eosinofílica crónica: ¿fenómeno autoinmune o enfermedad inmunoalérgica? Reporte de un caso y revisión de literatura. Reumatol Clin. 2012;8:145–8.