Pregnancy in women with autoimmune rheumatic diseases is associated with several maternal and foetal complications. The development of clinical practice guidelines with the best available scientific evidence may help standardize the care of these patients.
ObjectivesTo provide recommendations regarding prenatal care, treatment, and a more effective monitoring of pregnancy in women with lupus erythematosus, rheumatoid arthritis (RA) and antiphospholipid syndrome (APS).
MethodologyNominal panels were formed for consensus, systematic search of information, development of clinical questions, processing and staging of recommendations, internal validation by peers and external validation of the final document. The quality criteria of the AGREE II instrument were followed.
ResultsThe panels answered 37 questions related to maternal and foetal care in lupus erythematosus, RA and APS, as well as for use of antirheumatic drugs during pregnancy and lactation. The recommendations were discussed and integrated into a final manuscript. Finally, the corresponding algorithms were developed. In this second part, the recommendations for pregnant women with RA, APS and the use of antirheumatic drugs during pregnancy and lactation are presented.
ConclusionsWe believe that the Mexican clinical practice guidelines for the management of pregnancy in women with RA and APS integrate the best available evidence for the treatment and follow-up of patients with these conditions.
El embarazo en mujeres con enfermedades reumáticas autoinmunes se asocia a diversas complicaciones materno-fetales. El desarrollo de guías de práctica clínica con la mejor evidencia científica disponible puede ayudar a homogeneizar la atención en estas pacientes.
ObjetivosProporcionar recomendaciones respecto al control prenatal, el tratamiento y el seguimiento más efectivo de la mujer embarazada con lupus eritematoso sistémico, artritis reumatoide (AR) y síndrome por anticuerpos antifosfolípidos (SAF).
MetodologíaPara la elaboración de las recomendaciones se conformaron grupos nominales de expertos y se realizaron consensos formales, búsqueda sistematizada de la información, elaboración de preguntas clínicas, elaboración y calificación de las recomendaciones, fase de validación interna por pares y validación externa del documento final teniendo en cuenta los criterios de calidad del instrumento AGREE II.
ResultadosLos grupos de trabajo contestaron las 37 preguntas relacionadas con la atención materno-foetal en lupus eritematoso sistémico, AR y SAF, así como de fármacos antirreumáticos durante el embarazo y lactancia. Las recomendaciones fueron discutidas e integradas en un manuscrito final y se elaboraron los algoritmos correspondientes. En esta segunda parte se presentan las recomendaciones para mujeres embarazas con AR, SAF y el uso de fármacos antirreumáticos durante el embarazo y lactancia.
ConclusionesLa guía mexicana de práctica clínica para la atención del embarazo en mujeres con AR y SAF integra la mejor evidencia disponible para el tratamiento y el seguimiento de estas pacientes.
Below is the second part of the clinical practice guidelines for pregnancy care in women with autoimmune rheumatic disease of the Mexican College of Rheumatology, which has been divided into two parts. The first part should be consulted as regards development and methodology.
Rheumatoid ArthritisRheumatoid arthritis (RA) is a systemic inflammatory disease characterized mainly by inflammation and destructive proliferation of the autoimmune synovial membrane. Frequency of RA increases with age, but it tends to affect women since their reproductive stage.
In Women With RA, Which is the Disease Effect and Treatment Regarding Fertility and Fecundity?There is no evidence of the effects on the fertility rate in women with RA. A secondary infertility rate in women with RA was identified in Mexico, which is the same as the rate reported in the general population (20%).1,2[LOE III] According to a case control study, no differences have been found in the annual pregnancy incidence in women with RA as compared to parity in women without RA.1[LOE III] The obstetric and gynaecological history shall be considered in the comprehensive assessment of women with RA.1[GR C]
- •
In patients with RA, it is important to identify the obstetric and gynaecological history and assess parity in particular. [GPP]
Barrier methods shall be used with spermicides for decreasing the risk of pregnancy. The intrauterine device (IUD) is an effective method in 95% of the cases. The morning-after pill is effective in 99% of the cases.3[LOE III] Contraceptives with oestrogens and with progestogens alone are effective in 95% of women with RA. The first ones decrease the secondary effects of progesterone. When used with diaphragms and cervical caps, its efficacy reaches 98%.3[LOE III] The contraceptive methods based on progestogens alone, which are available for oral administration, injectable solution or subcutaneous implants are not associated to relapses of the disease, nor to the excess in the risk of thrombosis in patients with RA.3[LOE III] The three main recommended contraceptive types for women with RA are barrier methods, oral contraceptives with progestogens alone and the intrauterine device.3[GR C] The three year contraceptive implant is an effective option for women with RA. The intrauterine device is another option for long term use up to 5 years. The morning-after pill may be used in patients with RA.3[GR C]
- •
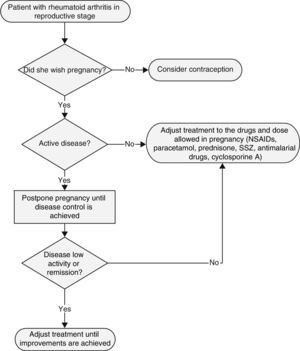
According to the clinical and therapeutic context, contraceptive counselling should be provided and, in accordance with the case and preferences, the best contraceptive method should be indicated in all patients with RA in childbearing age (Fig. 1). [GPP]
The use of different contraceptive methods, including barrier methods, IUD, subcutaneous implant, oral contraceptives based on progestogens alone or the ones containing oestrogen, is not associated with a complication rate increase, nor with the disease relapse risk.3,4[LOE III] IUD expulsion rate following the first 20 days after insertion if of 5%. Method permanence rate after 1 year is of 80%.3,4[LOE III] The different contraceptive methods may be used by women with RA, as their security profile is similar to that for women without the disease.3,4[GR C]
- •
The contraceptive method should be chosen by consensus between the patient with RA and the treating physician, according to the case specific preferences, wishes and security profile. [GPP]
A prospective study, conducted in 84 pregnant women with RA, showed an improvement in the disease activity (DAS28 assessed) during pregnancy, with an increase of the postpartum intensity of activity (P=0.035).5[LOE IIb] Complete disease remission is mainly reached in the third trimester of pregnancy. However, 57%–80% of patients presented improvements since the first trimester.6[LOE IV] During the postpartum period, there is a temporary risk increase of developing RA, or of RA exacerbation, mainly in the first 3–12 months.7[LOE IIb] Some studies have reported improvements of the disease activity in patients with RA during pregnancy (between 54 and 83%).8,9[LOE III] Pregnancy is not contraindicated in women with RA. The probability of a clinical improvement is high in a considerable percentage of patients.5–9[GR B/C] During puerperium, it is recommended to closely monitor the clinical course of the disease to detect exacerbations and perform the necessary therapeutic adjustments.7[GR B]
- •
RA is a disease which does not contraindicate pregnancy. Patients should receive the required medical counselling and should be aware that even when a disease improvement is highly probable during pregnancy, RA exacerbation is probable during puerperium. [GPP]
Cellular immune response regulation during pregnancy is one of the proposed factors which explain joint pain, arthritis and morning joint stiffness clinical improvements in pregnant patients with RA.10[LOE IIa] Th1–Th2 cytokines profile balance in pregnancy changes in favour of Th2, humoral immunity, which induces cytokine presence with anti-inflammatory effect; this is one of the other studied mechanisms involved in the clinical improvements of pregnant patients with RA.11[LOE IIa] HLA disparity between the mother and the foetus is another one of the mechanisms involved in the RA clinical activity improvement in pregnant women.12[LOE IIa] During the postpartum period, there is an increased risk of developing RA or an exacerbation; there are three probable explanations: microchimerism, as related to the role of foetal cells persistence in maternal circulation followed by the disease progression (RA), the effect of parity and the role of lactation.13[LOE IIa]
- •
The mechanisms involved in RA clinical improvements during pregnancy and exacerbation during the postpartum period are partially explained and should be extrapolated to our daily clinical practice. [GPP]
Applying the DAS28 instrument in normal pregnant women results in spurious additions to global score which has been estimated in 0.22 for the global health assessment, of 0.25 for CRP and of 1.1 for ESR.14[LOE IIb] From the four variants of the DAS28 instrument, the one which is performed with CRP and without the global health assessment component is the one which closely correlates with the real status of RA clinical activity during pregnancy.14[LOE IIb] The use of CRP DAS28 instrument without the global health component for clinical status assessment of the disease activity is recommended in pregnant women with RA.14[GR B]
- •
Clinical assessment of a pregnant woman with RA should include at least a quantification of the number of painful and swollen joints, CRP and ideally, scales of functional assessment and disease activity rate. [GPP]
Pregnancy by itself results in an additive spurious effect in the HAQ functional instrument qualification, with an estimated median of 0.5 during the third trimester.14[LOE IIb] Until case specific adapted variants are available, in pregnant women with RA, it is advisable to carefully consider the results of the HAQ qualification, since this can be overestimated, especially during the third trimester of gestation.14[GR B]
In Pregnant Women With RA, Which is the Predictive Effect of Antibodies (Rheumatoid Factor and Anti-cyclic Citrullinated Peptide Antibodies) on the Disease Activity?Simultaneous negativity for the rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibodies (anti-CCP) at the beginning of pregnancy is associated to a greater possibility of spontaneous improvements of the disease during pregnancy (75 vs 39%, P=0.01).15[LOE IIb] In patients with active RA at the beginning of pregnancy, it is recommended to determine the rheumatoid factor and anti-CCP, since seronegativity for both cases predicts spontaneous improvements of the disease.15[GR B]
- •
It is important to know the positivity status of the RF and the anti-CCP at the beginning of pregnancy in a woman with RA, as it allows to predict the clinical activity course of the disease during gestation. [GPP]
In women with seropositive RA to RF and anti-CCP, serum levels of these antibodies remain unmodified as regards the preconception levels and are not associated to changes in the clinical activity status of the disease.15[LOE IIb] Serial determination of the RF and anti-CCP levels during pregnancy in women with RA is not recommended, because their levels do not change as regards the preconception status; they remain stable during the course of gestation and are not associated with changes in the clinical activity of the disease.15[GR B]
- •
Serial determination of the RF and anti-CCP levels is not required during pregnancy of a woman with RA. [GPP]
An increased risk of low birth weight (OR 1.47; 95% CI 1.22–1.78), short stature for the gestational age (OR 1.20; 95% CI 1.05–138), preeclampsia (OR 2.22; 95% CI 1.59–3.11) and delivery via caesarean section (OR 1.19; 95% CI 1.07–1.31) have been observed in pregnant women with active RA.16[LOE IIb] In births from patients with RA who used prednisone, the gestational age was significantly lower (38.8 WOG vs 39.9 WOG; P=0.001) and preterm births have been more frequently observed (8.6 vs 6.2%; P=0.004) compared to births from women of the general population.17[LOE IIb] In women with RA, a greater risk of preterm pregnancies (9.2 vs 6.2%), more frequency of low birth weight (5.9 vs 3.6%) and deaths (0.9 vs 0.4%) have been observed compared to the general population.18[LOE IIb] As it seems there is an increased perinatal risk, especially if there is active disease, it is recommended to perform an adequate control of the RA before planning a pregnancy.16[GR B]
- •
In women with RA who wish to become pregnant, an adequate control of the disease is important, in order to get medical counselling and determine if it is appropriate or not to become pregnant (Fig. 1). [GPP]
In animal models, non-steroidal anti-inflammatory drugs (NSAID) at suprapharmacologic doses are teratogenic if administered at early stages of gestation.19[LOE IV] In animal models and in humans, NSAIDs administered during late stages of gestation may produce premature closure of the ductus arteriosus and pulmonary hypertension in newborns.19[LOE IV] No serious effects of glucocorticoids have been described when used in low or medium doses during pregnancy.19[LOE IV] Prednisone, prednisolone and methylprednisolone do not cross the placental barrier.19[LOE IV] There is contradictory information as regards congenital malformations and gestational miscarriage in women with RA exposed to methotrexate in an unnoticed way during pregnancy. No increased risk has been found in a systematic review of publication bias, while a postal survey reported congenital malformations in pregnant women with RA who were exposed to DMARDs only in those women with unnoticed exposure to methotrexate.20,21[LOE III] Methotrexate and leflunomide are classified as category X drugs (Table 1) according to the Food and Drug Administration (FDA) classification as regards drug teratogenicity.22[LOE IV] Hydroxychloroquine and sulfasalazine are not associated to an increase on the rate of spontaneous abortions, foetal or perinatal deaths, preterm birth or birth defects.22,23[LOE IV] Tumour necrosis factor antagonists are classified as category B drugs by the FDA (Table 2).24[LOE IV] Rituximab and tocilizumab are classified as category C drugs by the FDA (Table 2).24[LOE IV] In general, there is still not enough evidence about security of biological DMARDs during pregnancy.24[LOE IV] It is recommended to avoid the use of NSAIDs during pregnancy, especially during the first trimester because of the teratogenicity risk, and in the last trimester because of the risk of premature closure of the ductus arteriosus and obstetric complications, such as uterine contractile dysfunction or abnormal uterine bleeding.25[GR D] Glucocorticoids in low/medium doses (10–20mg/day of prednisone or its equivalent), in particular prednisone, prednisolone or methylprednisolone are the preferred drugs for symptomatic control of moderate to severe reactivations during gestation in patients with RA.22[GR D] Due to their proven security, hydroxychloroquine and/or sulfasalazine are the only DMARDs recommended as the initial or maintenance therapy during pregnancy in patients with RA.22,26[GR D] Methotrexate and leflunomide are contraindicated during pregnancy and their use should be immediately interrupted as soon as the unexpected concurrence is detected.19,22,25[GR D] The use of biological DMARDs during pregnancy is not recommended because the current evidences about their security are still limited. In case of pregnancy during treatment with biological therapy, treatment shall be discontinued.24,27,28[GR D]
- •
Women of childbearing age with RA, in particular those who wish to become pregnant, shall be informed about the best time for planning a pregnancy, the characteristics of the need and duration of contraception, the maternal and foetal risks in case of an unexpected pregnancy, and the RA risks of relapse or improvement during pregnancy and puerperium, as well as drug safety and efficacy, especially of DMARDs during gestation and breastfeeding period (Fig. 1). [GPP]
Drug Classification During Pregnancy, According to the US Food and Drug Administration (FDA).
| Category in pregnancy | Description |
|---|---|
| A | Adequate and well controlled studies in pregnant women have not shown risk to the foetus in the first trimester of pregnancy; however, there is no evidence of risk in the last trimesters |
| B | Studies on animal reproduction have not shown an adverse effect on the foetus, but there are no adequate and well controlled clinical studies performed in pregnant women or animals that had shown an adverse effect. |
| C | Studies on animal reproduction have shown an adverse effect on the foetus, but there are no adequate and well controlled studies performed in human beings; however, the potential benefits allow the use of the drug in pregnant women in spite of its potential risks. |
| D | There is evidence of risk to the foetus based on research data, post-marketing data, adverse reaction records or studies performed in humans, though the potential benefits of its use in pregnant women may be acceptable in spite of the probable risks. |
| X | Studies performed in animals or in humans have shown foetal abnormalities and/or the existence of evidence of risk to the human foetus based on adverse reaction records obtained from investigations or from the market, and there are risks involved with the use of the drug in pregnant women which clearly exceed the potential benefits. The use of the pharmacological product is contraindicated in those women who are pregnant or may become pregnant |
Breastfeeding in the postpartum period may exacerbate the disease in patients with RA, because of prolactin increase; it has been proven that this hormone promotes autoimmunity and pro-inflammatory response.29[LOE IIb] It is recommended not to contraindicate breastfeeding in women with RA, provided that they do not require drugs which are incompatible with breastfeeding; it is recommended to explain the disease exacerbation to the patient.29[GR B]
- •
Breastfeeding may be allowed in patients with RA, provided that the treatment they receive does not contraindicate it. Patients should be informed about the probability of disease exacerbation so as to make a joint decision. [GPP]
Antiphospholipid antibody syndrome (APS) is a systemic autoimmune disease characterized by the presence of antiphospholipid antibodies and associated clinical manifestations such as arterial thrombosis, venous thrombosis and/or obstetrical complications, in particular recurrent gestational miscarriages.30
In Women With APS, Which Are the Safest Contraceptive Options?Combined oral contraceptives, patches and transdermic implants, the vaginal ring, as well as the monthly, bimonthly and quarterly injectable contraceptives are contraindicated in patients with systemic lupus erithematosus (SLE) and positive antiphospholipid antibodies (aPL).31,32[LOE Ib] There is no direct information obtained from patients with APS primary or secondary to SLE; therefore, the same recommendations for patients with SLE and positive aPL shall be followed. Therefore, combined oral contraceptives, patches and transdermic implants, the vaginal ring and the monthly, bimonthly and quarterly injectable contraceptives are not recommended in women with APS.31,32[GR A] Copper-releasing IUD is a safe method for women with APS. Levonorgestrel releasing IUD may be used in particular cases and under close medical supervision.31,32[GR A]
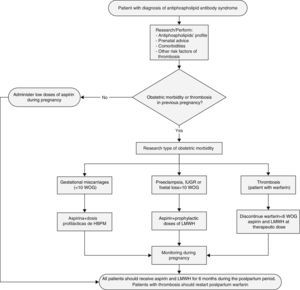
In Women With APS, Which Are the Actions and Procedures That Should Be Implemented During the Prenatal Control?High blood pressure and pulmonary arterial hypertension during pregnancy are associated to several maternal and foetal complications such as maternal death and gestational miscarriages.33,34[LOE IIa/III] It is suggested to avoid pregnancy in women with secondary APS and with history of thrombosis in the last 6 months, PAH, uncontrolled AHT or history of HELLP or serious preeclampsia.35[GR D] While planning pregnancy, it is recommended to perform the aPL profile and to get preconception advice about the risks of pregnancy complications (Fig. 2).35[GR D] As from the second trimester of pregnancy, the US Doppler may be useful as a predictor of preeclampsia and placental failure in patients with APS primary or secondary to SLE.36[LOE III] In pregnant women with APS, it is useful to perform a US Doppler of the uterine and umbilical artery since the second trimester and on a monthly basis to predict preeclampsia and placental failure.36[GR C] In pregnant women with APS, it is suggested to perform prenatal visits every 2 weeks until the middle of gestation and then weekly for early detection of urine protein, thrombocytopenia, BP and US obstetric control and flowmetry.37[GR D]
In Pregnant Women With APS, Which Are the Risk Factors to Develop Preeclampsia?The relative risk (RR) of preeclampsia in aPL carriers is of 9.72 (95% CI 4.34–21.75). Other risk factors in the general population to consider are the past history of preeclampsia (RR 7.19), pre-existing diabetes (RR 3.56), multiple pregnancies (RR 2.92), nulliparity (RR 2.91), family history (RR 2.9), high DBP (≥80mmHg) at the beginning of pregnancy (RR 1.38), high BMI before pregnancy (RR 2.47) and maternal age of ≥40 years (RR 1.96).38[LOE Ia]
In Pregnant Women With APS, With History of 3 r More Early Miscarriages (≤10 OG) Without History of Previous Thrombosis, Which Are the Most Effective Treatment Options (Fig. 2)?The combined therapy of aspirin at low doses (81–100mg/daily) plus heparin at prophylactic doses (unfractionated 5000IU every 12h or low molecular weight heparin) is more effective as compared with aspirin alone at low doses to reduce the gestational miscarriage rate (>50%).38,39[LOE Ia] A meta-analysis of five studies (334 patients with recurrent gestational miscarriages with positive aPL) showed a live birth rate of 74.2 vs 55.8% with heparin at prophylactic doses plus aspirin at low doses vs aspirin alone at low doses, respectively.40[LOE Ia] Treatment with heparin at prophylactic doses should be indicated after confirmation of pregnancy and continued during all the gestational period.39,40[GR A] The effect of adding prednisone in pregnant women with APS has not shown to be better than the regime with aspirin and/or heparin, and it actually increases the risk of complications (high blood pressure, gestational diabetes, prematurity).41[LOE Ib] In a series of 23 pregnancies in women with gestational miscarriages associated with treatment resistant aPL, addition of prednisolone 10mg during the first trimester to the regime of low doses of aspirin plus heparin improved the live birth rate (from historical 4 to 61%) but with a high risk of maternal and foetal complications.42[LOE III] It is not recommended to systematically add prednisone to the conventional treatment of APS in pregnant women.41[GR A] Treatment with intravenous immunoglobulin has not shown to be better than heparin and aspirin at low doses in women with APS and recurrent gestational miscarriages, with a live birth rate of 39.5 vs 72.5% (P=0.003), respectively.43[LOE Ib] The systematic use of intravenous immunoglobulin is not recommended in pregnant women with APS.43[GR A]
In Pregnant Women With APS, With a History of at Least One Foetal Death (>10 WOG) or Preterm Birth (<34 WOG) Due to Serious Preeclampsia or Placental Failure Without History of Previous Thrombosis, Which Are the Most Effective Treatment Options (Fig. 2)?The combined therapy of aspirin at low doses plus unfractionated heparin at prophylactic doses (5000IU every 12h) or low molecular weight heparin is more effective as compared with aspirin alone at low doses to reduce the gestational miscarriage rate (>50%).39,40[LOE Ia] Treatment with heparin must be initiated after the confirmation of pregnancy and continued during all the gestational period.39,40[GR A]
In Pregnant Women With APS, With History Previous Thrombosis, Regardless of Her Obstetric Medical History, Which Are the Most Effective Treatment Options (Fig. 2)?Pregnant patients with APS and history of previous thrombosis have been systematically excluded from controlled clinical trials.35[LOE IV] In pregnant women with APS and history of previous thrombosis, a therapeutic regime (secondary thromboprophylaxis) is recommended with low doses of aspirin plus low molecular weight heparin with anticoagulation doses (e.g., SC enoxaparin 1mg/kg or SC dalteparin 100U/kg, every 12h or SC enoxaparin 1.5mg/kg/day or SC dalteparin 200U/kg/day.35,44[GR D] It is recommended to add calcium supplements and vitamin D to the treatment, in order to decrease the osteopenia risk.35[GR D] The new oral anticoagulants such as dabigatran, rivaroxaban or apixaban are not recommended during pregnancy.45[GR D]
In Pregnant Women With APS, What Is the Influence of Antiphospholipid Antibodies in the Clinical Course and in the Therapeutic Decision?The aPL may be associated with recurrent foetal loss. The lupus anticoagulant has been associated with preeclampsia (OR 2.34; 95% CI: 1.18–4.64), intrauterine growth retardation, (OR 4.65; 95% CI 1.29–16.71) and late foetal loss (OR 4.73; 95% CI 1.08–20.81).46[LOE Ia] Anticardiolipins have been associated with preeclampsia (OR 1.52; 95% CI 1.05–2.20) and late foetal loss (OR 4.29; 95% CI 1.34–13.68).46[LOE Ia] Anti-B2GP1 antibodies have been associated with preeclampsia (OR 19.14; 95% CI 6.34–57.7), intrauterine growth retardation, (OR 20.03; 95% CI 4.59–87.43) and late foetal loss (OR 43.46; 95% CI 1.21–456).46[LOE Ia] The lupus anticoagulant is a primary predictor (RR 12.5; 95% CI 1.27–50.54) of the adverse result in pregnancy after 12 weeks of gestation.47[LOE IIb] There are no stratified controlled clinical trials that have assessed the pregnancy treatment according to the antiphospholipid profile.48[LOE IV] In pregnant women with APS, the aPL profile determines a risk in pregnancy, but not the treatment regime.44[GR D]
In Women With APS, Which Are the Most Effective Treatment Options in the Puerperium?During the first 6 weeks of the postpartum period, women have an elevated risk of presenting venous thromboembolism.48[LOE Ia] In women with obstetric APS, it is recommended to continue with low molecular weight heparin during 6–12 weeks of the postpartum period and in those patients with history of thrombosis, warfarin administration shall be initiated orally, both drugs being safe during breastfeeding.49[GR B]
In Women With APS, Which Are the Safest Peripartum or Peri-caesarean Treatment Options?In women with APS, it is recommended to discontinue the use of low molecular weight heparin 12h before the surgical procedure or at the beginning of labour and aspirin shall be discontinued at least 7 days before the surgical procedure.35[GR D]
In Children of Women With APS, Which Are the Actions and Procedures that Should Be Implemented During the Neonatal Follow-up?Neither thrombosis nor SLE was found in a 5-year follow-up of children of women with APS. There have been reports of children with disruptive behaviour disorders (autism, hyperactivity disorder, feeding disorder with language retardation and axial hypotony with psychomotor retardation).50[LOE III] Follow-up is recommended in newborns from mothers with APS, in search of disruptive behaviour disorders.50[GR C] Perinatal thrombosis in children from mothers with APS is a rare event with fatal outcome.51[LOE IV] It is recommended to perform the aPL profile in newborns from mothers with APS and clinical evidences.51[GR D]
Antirheumatic DrugsPlanning for pregnancy in a woman with rheumatic disease requires a careful analysis of drugs in order to keep the disease under control and minimize the risks for the foetus. The information about the use of drugs during pregnancy is limited and largely derives from their unnoticed exposure during unplanned pregnancies (Table 1).
In Pregnant Women With Autoimmune Disease, What Is the Risk of Foetal Exposure to Non-steroidal Anti-inflammatory Drugs or Analgesics (NSAIDs)?The use of aspirin during the first trimester of pregnancy increases the risk of gastroschisis in new-born (OR 2.37, 95% CI 1.44–3.88).52[LOE Ia] The use of NSAIDs during the first trimester of pregnancy increases the risk of congenital cardiac malformations (OR 1.86).53[LOE Ia] The exposure to NSAIDs during the third trimester of pregnancy increases the risk of premature closure of the ductus arteriosus.54[LOE III] To avoid the risk of premature closure of the ductus arteriosus, the use of NSAIDs is not recommended as from the week 32 of gestation.54[GR C]
A population-based cohort study concluded that prenatal use of NSAIDs increases the risk of abortion by 80% (HR 1.8; 95% CI 1.0–3.2).55[LOE III] The use of NSAIDs during the third trimester of pregnancy is associated with renal dysgenesis and oligohydramnios.56[LOE III] Administration of aspirin in doses lower than 100mg per day is safe during pregnancy.57[GR D] All NSAIDs, except for aspirin at low doses, should be discontinued before 32 weeks of gestation.57[GR D]
In Pregnant Women With Autoimmune Disease, What Is the Maternal and Foetal Risk of Exposure to Glucocorticoids for the Treatment of the Disease?Population-based cohort studies showed no significant association between the use of non-fluorinated glucocorticoids during pregnancy and the development of orofacial malformations (cleft lip and palate).58,59[LOE IIb] Non-fluorinated glucocorticoids at required doses may be administered during pregnancy to control the disease.58[GR B]
In clinical trials, a single administration of fluorinated glucocorticoids in pregnant women with signs of preterm birth was associated with a significant reduction in the rate of neonatal death, respiratory distress syndrome and intraventricular haemorrhage.60[LOE Ia] In clinical trials, the administration of multiple courses of fluorinated glucocorticoids before birth was not associated with improvements in neonatal mortality.61[LOE Ia] In women with risk of preterm birth between weeks 24 and 34 of gestation, it is recommended to indicate a single course of prenatal fluorinated glucocorticoid, if necessary.61[GR A] Prenatal exposure to glucocorticoids is not related to the presence of sepsis in the newborn.62[LOE III] When glucocorticoids are required for the treatment of the autoimmune disease in the mother, it is recommended to use prednisone, prednisolone or methylprednisolone at the lowest possible dose and time.62[GR C]
In pregnant women taking glucocorticoids during pregnancy, the risk of developing diabetes mellitus, high blood pressure and osteoporosis is the same as the risk in non-pregnant women.63[LOE III] Pregnant women who require chronic use of glucocorticoids shall receive the same recommended measures than other users of these drugs, for the prevention of diabetes, overweight, dyslipidaemia and osteoporosis.63[GR C]
In Pregnant Women With Autoimmune Disease, What Is the Risk of Foetal Exposure to Antimalarials, Azathioprine, Sulfasalazine, Cyclosporine A, Leflunomide, Mycophenolic Acid, Cyclophosphamide and Methotrexate?The use of antimalarial drugs during pregnancy is not associated with congenital malformations, spontaneous abortions, foetal death or prematurity.64[LOE Ia] The use of antimalarial drugs during pregnancy in patients with SLE is associated with a decreased activity of the disease, a lower rate of relapse and it allows lowering the dose of prednisone.65[LOE Ib] The administration of antimalarial drugs is safe during pregnancy.64[GR A]
There is not a significant association between the use of sulfasalazine and congenital malformations, foetal death, abortion, preterm births or low birth weight.66,67[LOE Ia] Findings in observational studies do not document a significant increase in the prevalence of congenital malformations in children of those women treated with sulfasalazine during pregnancy.68,69[LOE III] Pregnancy is safe in women treated with sulfasalazine. Due to its mechanism of action, it is recommended to administer folic acid as a supplement.57,69[GR A]
The use of azathioprine and 6-mercaptopurine during conception and pregnancy is not associated with congenital malformations or low birth weight, though it is associated with preterm birth.70[LOE Ia] The exposure of men to thiopurines at conception is not associated with congenital malformations. [LOE Ia] Azathioprine may be used during pregnancy to control the autoimmune disease.71[GR A]
The use of cyclosporine A during pregnancy is not associated with congenital malformations, preterm birth or low birth weight.72[LOE Ia] If necessary, cyclosporin A may be used during pregnancy.72[GR A]
In animals exposed to leflunomide, there is a high risk of teratogenicity and embryo death. However, studies in humans have shown no teratogenic effects.57,73,74[LOE III/IV] A prospective analysis of 64 pregnant women with RA receiving leflunomide during the first trimester of pregnancy (95% received cholestyramine) found no differences in major structural defects of newborns compared to women not exposed to the drug or healthy women (5.4 vs 4.2%, P=0.13).73[LOE IIb] The use of leflunomide is not recommended during pregnancy.57,75[GR D] Patients (men and women) treated with leflunomide and planning pregnancy should discontinue leflunomide 2 years before, or perform a disposal protocol (cholestyramine 8g every 8h for 11 days by measuring serum levels and, if required, repeating the scheme of cholestyramine).57,73–75[GR D]
Maternal exposure to mycophenolic acid during the first trimester of pregnancy is associated with increased foetal loss and a characteristic embryopathy (microtia, cleft lip and palate, brachydactyly and involved organs such as heart, kidney and central nervous system).76,77[LOE IV] If planning a pregnancy, it is recommended to discontinue mycophenolic acid three months before conception.76–78[GR D]
The administration of methotrexate during pregnancy is associated with increased incidence of abortions.79[LOE IV] Methotrexate is a teratogenic drug associated with aminopterin syndrome (intrauterine growth retardation, frontal bone hypoplasia, abnormal ossification of skull, low set ears, micrognathia, limb abnormalities and cardiac disorders).56[LOE IV] The use of methotrexate is contraindicated during pregnancy.56[GR D] Methotrexate has a long average life and is widely deposited in maternal tissues for up to 4 months.63[LOE IV] Because of the long average life of methotrexate, its use should be discontinued 3–6 months before conception.79[GR D]
Cyclophosphamide is associated with early menopause, nulliparity, premature ovarian failure, impaired spermatogenesis and decreased frequency of pregnancies.80[LOE IIb] Cyclophosphamide is gonadotoxic in men and women. In women, it depends on the cumulative dose and age, while in men there is no threshold of cumulative dose.57[LOE IV] The administration of cyclophosphamide during the first trimester of pregnancy is associated with embryopathy.57[LOE IV] Cyclophosphamide should not be used during pregnancy, especially in the first trimester.57,63[GR D] In premenopausal women with no parity satisfied, it is suggested to preserve ovarian function with the use of antagonists of gonadotropin releasing hormone.81[GR D]
There is no increased risk of abortion and congenital malformations with the use of tacrolimus.82[LOE III] The available evidence suggests that tacrolimus may be used during pregnancy.82[GR C]
In Pregnant Women With Autoimmune Disease, What Is the Risk of Foetal Exposure to Biological Drugs (Anti-TNFα, Rituximab and Other) (Table 2)?In a systematic bibliography review about women with inflammatory bowel disease (IBD) exposed to TNFαblocking agents (infliximab, adalimumab, certolizumab pegol) it was found that the rate of spontaneous abortion and congenital abnormalities is similar to the rate for the general population or women with IBD not exposed to these drugs.83[LOE Ib] A possible causal effect of in-utero exposure to TNFα antagonists (infliximab and etanercept) and the VACTERL association (vertebral abnormalities, anal atresia, cardiac defects, tracheoesophageal, renal and limb abnormalities) has been suggested in a US FDA database review.28[LOE III] In a European population-based study, it was unable to confirm the ratio of TNFα antagonists with the association of VACTERL in women with various autoimmune diseases.84[LOE III] Recently, in a prospective study in 86 pregnant women (mainly with RA IBD) exposed (97.6% in the first trimester) to TNFα blocking agents, a major risk of teratogenicity was not found compared to unexposed controls, and no cases of VATER/VACTERL association was found.85[LOE IIb] The use of biological agents during pregnancy is not recommended, although considering risk and benefit, if the patient is already receiving them, she can be kept up to week 20 of gestation.86–88[GR C]
A retrospective analysis of 153 pregnancies with maternal exposure to rituximab found 21% of abortions, 90 live births (76% at term) and 2% of congenital abnormalities.89[LOE III] The experience with the use of rituximab, abatacept and tocilizumab is limited as to draw conclusions on security in pregnancy.86[LOE IV] It has been shown that abatacept crosses the placenta. Certolizumab lacks the Fc portion of the antibody; however, the Fab fragment can cross the placenta passively at low levels during the first trimester.90[LOE III] Abatacept, certolizumab, rituximab or tocilizumab shall not be started or continued during pregnancy until more information becomes available.86[GR D] With abatacept, the use of contraceptive methods is recommended until 10 weeks after drug discontinuation.86[GR D].
In Women With Autoimmune Disease and Who Are Breastfeeding, Which Are Safest Antirheumatic Drugs That Can Be Used (Table 3)?Most NSAIDs are excreted in small amounts in maternal breast milk.91[LOE IV] The dose of NSAIDs is recommended immediately after breastfeeding in order to minimize the exposure of the baby57,91,92Table 3. [GR D]
Antirheumatic Drugs in Pregnancy and Breastfeeding.
| Drug | FDA category | Compatible with breastfeeding |
|---|---|---|
| Glucocorticoids | B | Yes, breastfeed 4h after the last dose |
| Non-steroidal anti-inflammatory drugs | B | Yes, with potential increase in the risk of jaundice and kernicterus |
| COX-2 inhibitors | C | Insufficient data |
| Hydroxychloroquine | C | Yes |
| Methotrexate | X | No |
| Leflunomide | X | No |
| Sulfasalazine | B, D | Yes, with precaution |
| Azathioprine | D | No |
| Cyclophosphamide | D | No |
| Cyclosporine A | C | No |
| Mycophenolic acid | C | No |
| Anti-TNF agents α | B | Insufficient data, No |
| Anakinra | B | Insufficient data, No |
| Rituximab | C | Insufficient data, No |
FDA, Food and Drug Administration.
The concentrations of prednisone and prednisolone are very low in breast milk even at doses greater than 20mg per day.63[LOE III] Women with autoimmune disease receiving glucocorticoid may continue breastfeeding.57,86,92[GR C] It is recommended to breastfeed 4h after receiving the dose of glucocorticoid or to take it immediately after breastfeeding.63[GR C]
The excretion of 6-mercaptopurine is low in breast milk. Non analytical observational studies conclude that the use of thiopurines during breastfeeding is safe.93–95[LOE IV] The use of azathioprine and 6-mercaptopurine appears to be safe during breastfeeding.88,93,96[GR D]
There is not enough evidence regarding the security of cyclosporin A during breastfeeding.57[LOE IV] The use of cyclosporine during breastfeeding is not recommended.57,92[GR D]
There is not enough evidence regarding the security of leflunomide during breastfeeding.57[LOE IV] Given the limited evidence regarding the security of leflunomide during breastfeeding, its use is not recommended.86,92[GR D]
There is no evidence in humans regarding the use of mycophenolic acid during breastfeeding.76,78[LOE IV] The use of mycophenolate mofetil during breastfeeding is not recommended.76,78[GR D]
Methotrexate has a long average life and is widely deposited in maternal tissues for up to 4 months.63,86[LOE IV] The use of methotrexate is contraindicated during breastfeeding.78,86,92[GR D]
Cyclophosphamide is excreted in breast milk and may cause suppression of haematopoiesis in infants.57[LOE IV] Cyclophosphamide should not be used during breastfeeding.57,63,92[GR D]
There is little evidence (case reports) related to the use of anti-TNF antibodiesα, anti-TNF fusion molecules α, abatacept, rituximab and tocilizumab during breastfeeding.86,88,92[LOE IV] The use of biological agents is not recommended during breastfeeding until more information becomes available.92[GR D]
Maternal administration of coumarin and heparin is safe for the new-born who is breastfed.97[LOE IV] Coumarins or heparins can be used as anticoagulant during breastfeeding due to its low excretion in breast milk.97[GR C]
In Pregnant Women With Autoimmune Disease, What Is the Risk of Foetal Exposure to Oral Anticoagulants and Heparin?The use of coumarins during the first trimester (6–12 weeks) is associated with isolated congenital malformation and embryopathy related to warfarin.98[LOE Ia] Warfarin easily crosses the placenta due to its low molecular weight; thus, it is associated with intracranial haemorrhage.97[LOE IV] In utero exposure to warfarin has been associated with cleft lip and palate, choanal atresia or stenosis, microphthalmia, optic atrophy, cataract, coarctation of the aorta, situs inversus, bilobed lungs and limb hypoplasia.97[LOE IV] Warfarin must not be administered during the first trimester of pregnancy.97,98[GR D] Clinical trials have shown that the use of low molecular weight heparin during pregnancy allows decreasing recurrent foetal loss rate in patients with thrombophilia.99[LOE Ia] Maternal treatment with coumarin and heparin is safe for the new-born who is breastfed.97[LOE IV] The use of unfractionated heparin and low molecular weight heparin as the preferred anticoagulants during pregnancy is recommended, since due to its molecular weight it has no transplacental transmission.97[GR D]
Final ObservationsThis clinical practice guideline can be easy to disseminate and implement although it can be difficult to apply for several reasons within the context of our social and cultural environment. Patients have little education about the impact of pregnancy on autoimmune rheumatic diseases and at some levels no full access to health services exists. Furthermore, the limited knowledge of physicians who are not rheumatologists as regards the effect of pregnancy on the autoimmune disease and vice versa may also be a limitation for its whole implementation. This requires an intensive educational intervention in our population to generate a change that results in better care for this group of patients, so we are confident this paper will be of help as far as this change is concerned.
Ethical ResponsibilitiesProtection of people and animalsAuthors state that no experiments were performed on human beings or animals as part of this investigation.
Confidentiality of dataAuthors state that this article does not contain patient data.
Right to privacy and informed consentAuthors state that this article does not contain patient data.
Conflict of InterestThe Mexican College of Rheumatology received unrestricted educational support from the company UCB. The staff working at UCB had no interference with the information vested herein and did not participate in any meetings of the working group.
Please cite this article as: Saavedra Salinas MÁ, Barrera Cruz A, Cabral Castañeda AR, Jara Quezada LJ, Arce-Salinas CA, Álvarez Nemegyei J, et al. Guías de práctica clínica para la atención del embarazo en mujeres con enfermedades reumáticas autoinmunes del Colegio Mexicano de Reumatología. Parte II. Reumatol Clin. 2015;11:305–315.