Systemic sclerosis (SSc) is a connective tissue disease that usually affects women, with a male:female ratio of 1:4–10.
It was thought that there was a prohibitive risk of fatal complications in the pregnancies of patients with SSc. It is now known that the majority of these women undergo a normal progression of pregnancy if the right time is chosen and a close obstetric care is delivered. The obstetric risk will depend on the subtype and clinical stage of the disease, and the presence and severity of the internal organ involvement during the pregnancy.
The management of these pregnancies should be provided in a specialized center, with a multidisciplinary team capable of identifying and promptly treating complications.
Treatment should be limited to drugs with no teratogenic potential, except when renal crises or severe cardiovascular complications develop.
La esclerosis sistémica (ES) es una enfermedad del tejido conectivo poco común que afecta principalmente a mujeres (relación mujer:hombre de 4–10:1).
En el pasado se pensaba que existía gran riesgo de complicaciones fatales en los embarazos de pacientes con ES. Actualmente, se sabe que muchas de estas mujeres pueden llevar a buen término un embarazo si se elige el momento adecuado y se lleva monitorización obstétrica estrecha. El riesgo obstétrico dependerá del subtipo y la fase clínica de la enfermedad y de la presencia y la gravedad de la afección de órganos internos durante el embarazo.
El manejo del embarazo de las pacientes con ES debe realizarse en un centro de atención especializada, con un equipo multidisciplinario capaz de detectar y tratar las complicaciones tempranamente.
El tratamiento debe limitarse a fármacos sin potencial teratogénico, excepto en crisis renales y en complicaciones cardiopulmonares que pongan en peligro la vida de la madre.
Systemic sclerosis (SSc) or generalized scleroderma is a relatively rare connective tissue disease of unknown etiology affecting skin, joints, blood vessels, heart, lungs, gastrointestinal tract and kidneys. It has an incidence of 2–10 cases per million and prevalence of 150–300 cases per million worldwide. It mainly affects women, with a peak incidence between the fifth and sixth decades of life with a female to male ratio of 4–10:1; this ratio increases during reproductive age (15–50 years), in which the relationship can reach 15:1.1,2
Survival in SS has improved in recent years, mainly thanks to the introduction of angiotensin-converting enzyme inhibitors (ACEI) in the 1980s, which decreased complications and mortality due to renal crisis. Interstitial lung disease and pulmonary hypertension have replaced renal failure as the most common causes of morbidity and mortality from scleroderma.2–9 The improvement in the prognosis of the patients due to the greater knowledge of the disease as well as increased access to health services and treatment of SS complications has been accompanied by an increase in the number of women with this condition who seek and achieve pregnancy.
This article will discuss the effects of SS on pregnancy, the influence of vascular disease, inflammation and fibrosis on the health of the mother and fetus, and the effects of pregnancy on SS.
Pregnancy and Systemic SclerosisIn the past there were few reports of pregnancies in patients with SS due, in part, to the fact that the peak age of onset is between 45 and 55 years, and the fact that reports of early cases showed disappointing outcomes for both mothers and babies.10–14 25–30 years ago it was relatively common for doctors to recommend their patients with SS not to conceive and even consider abortion because of the supposed high risk of fatal complications for mother and children. This initial information, based on isolated case reports, was replaced by a series of retrospective and prospective case series which showed large proportion of women with SS who led successful pregnancy with little risk of serious complications when the patient and physician discussed the issue and chose an appropriate time for pregnancy, performing15–18 close obstetric monitoring.
Pregnancy in women with SS should be considered from the onset as a high risk pregnancy due to the increased risk of premature delivery and low birth weight for gestational age. In early pregnancy, each patient should be carefully assessed to establish the subtype of disease (diffuse or limited), the (early or late) phase and the extent and severity of damage to internal organs. Those patients with a duration of symptoms of less than 4 years (scleroderma in early stage), diffuse subtype, or anti-topoisomerase i or anti-RNA polymerase iii antibodies have a higher associated obstetric risk and, if possible, should delay pregnancy until the patient is in a late stage and hence has a less active disease.15
The third trimester of pregnancy is considered the one with the highest risk, since the patient may develop complications secondary to hypertension, renal failure, pulmonary hypertension, interstitial lung disease or heart failure.19,20
Fertility in Systemic SclerosisIt has been difficult to establish whether fertility is affected in patients with SS, as studies have shown conflicting results. An Italian report made several decades ago suggested that there was no direct association of the disease with decreased fertility and conception problems and concluded that these issues should not be attributed to the SS.21 On the other hand, in two English studies, the authors postulate that fertility may be impaired in patients even years before the onset of the disease, with a twice greater risk of repeated spontaneous abortion and three times more fertility problems, defined by them as an unsuccessful pregnancy before 35 years of age.22,23
Steen et al. have examined this controversy in two retrospective studies in which they compared patients with SSc patients with rheumatoid arthritis (RA) and healthy women, and later performed a prospective study.
The first study demonstrated that patients who already had SS and who become pregnant have similar frequencies of spontaneous abortions (15%) than RA patients (16%) and healthy controls (13%), and conclude that the disease per se does not directly affect total fertility by increasing the proportion of abortions.16
In the second study, the pregnancy outcomes of women whose SS onset occurs during the childbearing years were compared with RA and healthy women. As for fertility, it showed that the percentage of women who had never been pregnant is higher in groups of patients with SS and RA than in healthy controls (P<0.05). However, this does not necessarily mean that patients have fertility problems, as there are additional factors that contribute to these differences. Among the study patients, 8%–10% had no sexual activity; women with SS or RA in this had developed the disease at a younger age compared to those sexually active; A questionnaire study showed that most women with SS (5%) and RA (11%) had chosen not to have children compared with the control group (3%), some of them motivated by the advice of doctors or family. Only 2%–5% of patients with SS had sought unsuccessfully to become pregnant; in most of them, the age of onset of SS was after 40, so they were already probably infertile.17
The study found no significant differences between groups in the number of women who reported a period of at least one year during which they failed to conceive (15% of patients with SS, 12% of patients with RA and 13% of controls). There were 27 women with SSc who were evaluated for infertility, and in that 63% of them already had the disease at the time of the evaluation; the rate of successful pregnancies in patients evaluated for infertility, regardless of the treatment they received for it, was similar in the three groups: 37% for patients with SS, 40% for patients with RA and 43% for healthy women. The analysis found no significant differences in the frequency of infertility or the rate of successful pregnancies in women with RA and SS before or after disease onset.17
Besides the above, this topic is especially related to the sexuality of patients, since vascular lung disease, skin and physical limitations, as well as changes in the appearance and the emotional effects of the disease, have the ability of impact relationships of some of the patients, thus affecting the possibility of conception. The main symptoms that patients reported as affecting their sex life were fatigue, muscle/joint pain, vaginal dryness and dyspareunia. They also associated Raynaud's phenomenon, sore hands, digital ulcers, dyspnea and chest pain.24–26
Patients may also have coexisting secondary antiphospholipid antibody syndrome (APS), so it would be appropriate to identify lupus anticardiolipin antibodies, β2 glycoprotein 1 and lupus anticoagulant in all patients with SS and recurrent fetal loss. In a study by Steen et al. antiphospholipid antibodies were found in 50% of patients with SS and ulcers of the lower limbs, indicating that the association of SS and APS cannot be that uncommon.27 There are other studies indicating that the antiphospholipid antibodies may be independently associated with pulmonary arterial hypertension, macrovascular disease and increased total mortality in patients with systemic sclerosis.28,29
Effects of Pregnancy on Systemic SclerosisIt is difficult to determine the effects of pregnancy on the disease, as some symptoms are very similar and, therefore, it is not easy to distinguish which of the causes are attributable. Such is the case of GERD, joint pain and swelling, to name a few. The consensus of several studies on this is that there are no significant changes in the disease during pregnancy.10,30 The prospective study by Steen et al. showed that the disease was stable in 60% of the patients, 20% noted improvement, particularly of Raynaud's phenomenon, and 20% worsening, particularly with regard to reflux, arrhythmia, arthritis, skin thickening and development of renal crisis in two cases.18
After delivery, 33% of women noticed an increase in the severity of some symptoms, particularly: Raynaud's phenomenon, arthritis, skin thickening and renal crisis. The 10-year survival of SS patients with and without pregnancy is similar. Most women did not have worsening of symptoms after delivery.18
Pulmonary arterial hypertension is a complication that gets worse during and after pregnancy and in which another pregnancy is not recommended because of the high mortality of this group of patients during pregnancy.31
Table 1 summarizes the changes in clinical signs and symptoms of organ systems in patients with SS during pregnancy.
Effects of Pregnancy on SS.
| General | The disease remains stable |
| Raynaud's phenomenon | Improvement during pregnancy. It may worsen after delivery, especially in case of complications |
| Skin disorder | It may start during pregnancy, and in some cases there may be DSS progression during the postpartum |
| Joints | Joint pain, similar to pregnancy in patients without SSThere may be arthritis |
| Gastrointestinal | Reflux, nausea, vomiting and constipation. Similar to patients without SSSpecialized care is needed in handling cases of poor intestinal absorption |
| Cardiopulmonary | Symptoms associated with cardiopulmonary conditions aggravated by SS. Management should be equal to that of pregnant patients with cardiopulmonary diseases without SSPoor prognosis in patients with PAH |
| Renal | Renal crises occur more frequently in early diffuse SS patients with or without pregnancy. Due to the high mortality, management with ACE inhibitors should be started early on suspicion of this complication, despite the fetal riskNo evidence of increased incidence of renal crisis has been seen in pregnancies |
SS: systemic sclerosis; PAH: pulmonary arterial hypertension; ACE inhibitors, angiotensin converting enzyme inhibitors.
Raynaud's phenomenon and vascular disease. Generally these symptoms improve during pregnancy, presumably by vasodilation associated with it.31 It is common for patients to report exacerbation in the postpartum period, who therefore need to restart treatment at this early stage, particularly those who have had previous ulcers or amputations.
Skin condition. In general, case series report that skin manifestations are usually stable during pregnancy, however there are some reports of the onset of SS during pregnancy. As these are isolated cases, it is not possible to determine whether SS develops more often during pregnancy.15 In the series by Steen and Medsger there were some diffuse SSc patients who worsened in their skin manifestations during pregnancy; in this period, patients had discontinued treatment and exacerbation could also be triggered by this factor.18
Joint pain and arthritis. Joint pains are common in all pregnant women, but there is no evidence that it is more frequent in patients with SS, although it is a common complaint; the physician must also assess whether there is arthritis because it can improve with treatment, as will be discussed later in this text.18
Gastrointestinal. Nausea, vomiting and reflux are more frequent and severe in patients with SS than in healthy pregnant women; poor intestinal absorption due to intestinal dysmotility and bacterial overgrowth can be a serious problem that can threaten the life of the patient and the baby, so its management should be continued throughout pregnancy. Another common problem is constipation.18,32,33
Pulmonary Fibrosis. Evidence in the literature has shown no decline in lung function in patients with mild or no fibrosis.18,31 In mild cases of pulmonary fibrosis, there are reports of successful outcomes with proper handling, but in five cases of women with severe pulmonary fibrosis, forced vital capacity (FVC)<65% was reported by Steen et al.; there were three preterm infants, an abortion and one maternal death from multiple organ failure as well as an18 elective abortion. In addition, dyspnea is a common complaint, which increases particularly during the third quarter of pregnancy.
Pulmonary arterial hypertension. PAH is a serious complication that occurs primarily in patients with SSc and limited long-term evolution of the disease and has been associated with the presence of anti-centromere antibodies, anti-Th/To and anti-U3 RNP (the last 2 with nucleolar pattern on immunofluorescence). However, it can also occur in patients of reproductive age, so there is the risk of concomitant PAH during pregnancy. Women with PAH who become pregnant may develop severe hemodynamic complications due to the low reserve of the pulmonary arterioles, which are not able to handle the increased blood volume and cardiac output occurring physiologically during pregnancy.31
Some studies in pregnant women with PAH, which included patients with PAH from various causes, estimated that maternal mortality ranges from 17 to 50%, with increased risk during labor and in the first 2 weeks postpartum, due to acute cardiovascular collapse.34–36
An analysis of pregnancy outcomes of women with primary PAH showed that these patients have an increased risk of hospitalization and hypertensive disorders of pregnancy.37 Multiple case reports and retrospective studies conducted both before and after the introduction of vasodilators such as sildenafil, prostaglandin analogs and nitric oxide, show high mortality in this group of patients38–48; no controlled studies exist comparing mortality before and after the era of these new vasodilators, and patients with SS and PAH should avoid conceiving, and even can be offered to electively terminate the pregnancy.
Renal disease. Renal crisis is a syndrome characterized by acute, refractory hypertension, progressive proteinuria and acute renal failure associated with microangiopathic changes on the biopsy, with the appearance of “onion skin” lesions of the renal arteries by endothelial proliferation. It affects 5%–10% of Caucasian patients, especially those with diffuse cutaneous SS (DCSS) of rapid evolution in the first 5 years from the onset of symptoms attributable to the disease, with the presence of anti-topoisomerase i or anti-RNA polymerase iiiantibodies, and previous exposure to corticosteroids at doses equal to or greater than 15mg/day, all these in the presence or not of pregnancy.1,15,20 In Mexico, the frequency of renal crisis is likely to be lower; an analysis of complications in internal organs and autoantibodies in our cohort (in a national reference center), mainly in Mexican mestizos from a national referral center, found a prevalence of 2% of renal crisis in the entire cohort, including incident and prevalent cases of these and other patients with DCSS and LSS (Limited systemic sclerosis).49
If there is clinical suspicion, the clinician must perform general laboratory tests including serum creatinine, blood count to look for microangiopathic hemolytic anemia and/or thrombocytopenia, liver function tests for differential diagnosis of HELLP syndrome, as well as consider a urinalysis for proteinuria and other abnormalities.1,15,20
Cases have been reported in which the increase in blood pressure (BP) is not as marked (normotensive renal crisis), so it is necessary to make a clinical differential diagnosis with thrombotic thrombocytopenic purpura (TTP), in which microangiopathic hemolytic anemia, thrombocytopenia and kidney failure are also present. For this, it is useful to measure the plasma levels of the activity of the metalloproteinase enzyme ADAMTS13, since its function is decreased (<10%) or absent in most of TTP patients, while normal in patients with SS and renal crisis.50
Another important differential diagnosis is preeclampsia and eclampsia. In this case, the prevalence of proteinuria further support the diagnosis of preeclampsia and elevated plasma renin levels further support the diagnosis of scleroderma renal crisis.1
The management will be discussed later in the section.
Women with diffuse scleroderma should be advised to become pregnant after their disease has been stable for at least 6 months and the period of greatest risk of developing renal crisis has passed, which is usually 3–5 years after the start of symptoms attributable to the disease.31 The time of this recommendation is based on observations made on the natural history of the disease and published data, as there are no observational longitudinal prospective studies that provide information about it.
MicrochimerismLong term persistence of low levels of DNA from cells from a genetically different individual is called microchimerism. Currently, it is known that during pregnancy there is cellular exchange between mother and fetus through the placenta, with estimates of 2–6 fetal cells per milliliter of maternal blood in the second trimester of pregnancy and lower levels after delivery. However, they may be found in the circulation of up to 64% of healthy women, even decades after delivery.1,51
In the case of SS, the role of microchimerism in the pathogenesis of the disease is of interest because of the similarities the disease has with the graft-versus-host disease (GVHD). It has been considered that microchimerism has the ability to induce autoimmunity through the difference between the major histocompatibility complex (HLA) of the donor cells (fetus) and the host (parent). A prospective study compared the presence of male DNA in women with SSc and healthy controls years after the birth of sons, yielding higher levels in the first group.52 It is possible that certain HLA genes as well as the relationship between the host HLA and the microchimeric cells are determinants of the microchimerism final effect in the host, since there are studies showing enhanced compatibility of HLA Class II, but not in the class i of families with SS. It is proposed that increased compatibility leads to an increase in the exchange of cells through the placenta. These fetal cells may have the ability to react against class I antigens or other minor histocompatibility antigens, altering immunoregulatory mechanisms in the mother, resulting in autoimmunity.1,53,54
This theory does not explain the incidence of SS in nulliparous women and men, but it has been speculated that it could be associated with the presence of maternal cells in these patients. In a study comparing the association of birth order and number of pregnancies/deliveries with the development of SS, using as controls the sisters of affected patients, it was found that the risk of developing SS increased with the order of birth which corresponded to the patients (2.–5° born. OR 1.25, 6.–9° born. 2.22 OR 10.°–15° birth: OR 3.53). It is believed that this could be related to an increase in exposure to chimeric maternal cells of previous pregnancies. This may rely on the fact that researchers have found small amounts of Y chromosome DNA even in nulliparous women.51,54
Furthermore, we have demonstrated the presence of microchimeric maternal cells in men with SS. It has been suggested that microchimeric maternal cells are more aggressive or require less stimulus to activate. One study even found more risk of developing SS in nulliparous patients than in those with parity (OR 1.37).53,55
Systemic Sclerosis Effects on PregnancyStudies on the effects of SS in pregnancy are scarce. The groups of Dr. Steen15–18 and Dr. Black have published some22 retrospective and prospective case series that have analyzed this issue.
Abortion: Several studies have recently demonstrated that, although there is a slight increase in the percentage of abortions observed when pregnancy occurs after the onset of the disease with regard to pregnant patients prior to onset of symptoms (15% vs 11%), the incidence of abortions in patients with SS is not higher than the general population or to a control group of patients with RA.17
Placental pathology: Due to the high vascular affection existing in SS patients, research has been performed on whether these pathophysiological changes also affect the placental circulation. A study that analyzed three placentas showed that there is decidual vasculopathy with stromal fibrosis and chronic infarction of chorionic villi, even in the babies born without complications between weeks 34 and 38 of pregnancy.56 It has also been observed that there is placental mesenchymal dysplasia, foamy degeneration of endothelial cells with vascular obstruction, foci with decreased vasculature, corioangiosis foci, fibrinoid deposit material, chorioamnionitis and accelerated placental maturation in these patients.57,58 In 4 of the 13 cases reported in the study by Doss et al., placental abnormalities correlated with fetal growth retardation.58 These alterations are similar to those found in pregnancies complicated by pregnancy-induced hypertension and perhaps are responsible for fetal complications, such as intrauterine growth restriction and preterm birth.
Preterm delivery: The study by Steen et al. showed a marked increase in the frequency of preterm infants in patients with SS, with the most frequent complications occurring in patients who already had the disease at the time of pregnancy (15%) versus patients who become pregnant before the onset of the disease (8%). In this study, which had a control group of healthy persons and patients with RA, the RA patients also had a higher frequency of births following disease onset, indicating that the reasons for these outcomes may not be unique to SS. It is possible that this increase in the number of preterm births is secondary to the mother presenting a chronic disease rather than the disease per se.16 There is no current study has been able to find a higher frequency of pregnancy complications in patients with SS justifying premature births.16–18 It is important to note that although women with SS have a higher risk of preterm delivery, this risk is not large enough to discourage pregnancy.
Hypertensive complications: Although an increase in the frequency of hypertensive diseases during pregnancy has not been found, a study of epidemiological data centers including primary care found an increased risk of about four times in most studies controls. The authors associated this increase to a less than optimal monitoring of patients compared to tertiary centers where clinical care protocols37,59 are normally developed.
When BP increases, their origin must be distinguished to determine whether they are secondary to SS (renal crisis), an obstetric event (preeclampsia) or the appearance of overlap with another rheumatic disease like lupus erythematosus with renal involvement, which can be especially complex from the clinical standpoint. To differentiate whether the etiology is of obstetric origin, seizures, elevated transaminases or serum urate may be useful; however, if the diagnosis remains unclear, the usefulness of the measurement of plasma renin levels has been proven. In preeclampsia, serum renin level varies from low to normal while in SS renal crisis, plasma renin levels are elevated as a result of renal cortical ischemia. In situations where both scenarios are indistinguishable, immediate empirical treatment is indicated. If, due to any situation, a definite diagnosis is absolutely necessary to start treatment, a renal biopsy may be indicated.60
Effects on the Fetus1. Low birth weight: There is an increase in the frequency of low birth weight for gestational age compared to patients without disease (10% vs 2%, approximately). This result is indicative of intrauterine growth restriction during fetal development, which has been associated with the vascular disease developed in patients with SS. Term infants with low birth weight were observed more frequently in patients who become pregnant after the onset of SS (11%) compared to women who became pregnant before the onset of the disease (5%). Another article, although it is also retrospective but with better methodology, shows an OR for IUGR of 3.74, a statistically significant association. At this point it is highly recommended to seek association with secondary APS, as this and the presence of nephropathy with proteinuria are prognostic determinants of the fetal outcome.
2. Fetal death: Even though it is traditionally believed that there is an increased risk of this complication, studies in recent years have shown that there is no significant increase in cases of stillbirth compared with healthy controls (4% vs 1.4%–2%).17–20
3. Fetal heart block: The presence of anti-Ro/SSA and anti-La/SSB antibodies in maternal serum has been associated with the development of heart block in the fetus or newborn. The prevalence of these antibodies is of 12%–37% and 4%, respectively, in patients with systemic sclerosis.49,61,62 Complete heart block develops in 1%–2% of pregnancies in patients with these antibodies, but the frequency may be increased up to 10 times with recurrent pregnancies, so intentional close fetal surveillance for data indicative of this complication is recommended.
There is no evidence of increased frequency of birth defects in children of mothers with systemic sclerosis.
Management PlanIdeally, in pre-pregnancy planning, the clinician should perform a detailed assessment of the state of the patient and discuss with her the possible complications and the importance of high-risk obstetric monitoring. It is important that the maternal disease be stable before pregnancy to reduce the likelihood of both maternal and fetal obstetric complications. In addition, the severity of the patient organic involvement and antibodies present (topoisomerase, anti-centromere, anti-RNA polymerase iii, anti-Ro/SSA, anti-La/SSB, anticardiolipin, -β2 glycoprotein 1 lupus anticoagulant) should be documented, to treat and prevent complications adequately.
Here, we present the evaluation and management of these patients according to the different organ systems that may be affected.
Vasculopathy. In patients with Raynaud's phenomenon and/or digital ulcers, general measures to avoid sudden temperature changes must be taken. During pregnancy, most vasodilators are contraindicated, but they may be restarted just after delivery.18,31 5-phosphodiesterase inhibitors such as sildenafil have been used without significant adverse effects on the mother or fetus for the treatment of PAH during pregnancy, particularly in the second half,38,46 and recently in some open studies to delay intrauterine growth retardation and for treatment of preeclampsia,63,64 so theoretically they could be used in severe cases of vascular disease during pregnancy, although so far no studies corroborate this.
Skin. For progression of cutaneous manifestations, there are few treatment options since most drugs used for this purpose are contraindicated (cyclophosphamide, D-penicillamine, methotrexate, mycophenolate mofetil). There are reports of improvement in the skin and articular manifestations using IV immunoglobulin in SS patients65 and it is considered safe in pregnancy,66 but its usefulness is disputed.
Joint manifestations. For the treatment of SS associated arthritis during pregnancy, painkillers such as paracetamol may be used, as well as low doses of corticosteroids (less than 15mg/day of prednisone or equivalent), hydroxychloroquine or, failing this, chloroquine, at minimum necessary doses and for the shortest possible time, though they have not been associated with an increased rate of birth defects or complications of scleroderma.20,67,68 Non-steroidal anti-inflammatory drugs should be limited de to their association with oligohydramnios.
Gastrointestinal manifestations. Upper gastrointestinal symptoms such as nausea and reflux, antacids as magaldrate, proton pump inhibitors and histamine receptor blockers at the minimal effective dose can be used.20,69 Pregnancy should be discouraged in cases of serious disorders of intestinal absorption; however, in a series of patients, one patient with poor intestinal absorption had three fetal losses and managed two successful pregnancies at term with normal weight babies after antibiotic therapy and hyperalimentation for malabsorption.18
Pulmonary fibrosis. Pulmonary function tests with diffusion of carbon monoxide are recommended before pregnancy and often as needed, as indicated by the cardiopneumologist, discouraging pregnancy in cases of severe disease (FVC<50%).18 During pregnancyç immunosuppressants, such as cyclophosphamide or mycophenolate mofetil, are contraindicated, but have been used to try to stop the progression of pulmonary fibrosis; the use of low-dose corticosteroids and immunosuppressive treatment in the immediate postpartum period may be considered.
Pulmonary arterial hypertension. It is recommended to perform an echocardiogram before pregnancy and during any clinical deterioration data over the course of the same. We suggest that women with PAH do not get pregnant. In case of pregnancy, the patient should be treated in a tertiary level center, with close supervision by a cardiopneumologist and continue specific treatment for PAH as needed, particularly during the second half of pregnancy, during and after delivery.31 At this stage, phosphodiesterase 5 inhibitors including sildenafil, prostaglandin analogs such as iloprost or epoprostenol, nitric oxide and supportive therapy with calcium channel antagonists and heparin anticoagulation38,43–46 can be used. The use of endothelin receptor antagonists is contraindicated during pregnancy.47–49
Cardiac manifestations. As for heart disease, there may be new or worsening heart failure, which can be documented with echocardiography. It is recommended to avoid pregnancy in severe cases (ejection fraction<30%). In the few case reports of women with SS and heart failure who became pregnant, favorable outcomes but with preterm births were seen, with frequent monitoring and aggressive management during and after birth with drugs commonly used for heart failure70; in the case of electrocardiographically diagnosed conduction blocks, intervention is rarely required.
Renal disorders. As previously discussed throughout the text, the presence of any elevation in the BP with respect to previous measurements has to be considered potentially serious. Because it is a true medical emergency in which life is at stake both for the mother and baby, as well as renal function, treatment should not wait for diagnostic confirmation and consists of high doses of ACE inhibitors orally or IV. The most widely used has been enalapril, which should be continued indefinitely,15 despite the possibility of fetal toxicity (anhidramnios, renal atresia, pulmonary hypoplasia and fetal death), as the risk of maternal complications exceeds the fetal risk.
If a woman with a previous renal crisis becomes pregnant, you can try driving the BP down with other antihypertensives and closely monitoring creatinine and BP. However, if any of the two are raised, restarting the ACE inhibitor is indicated. In the prospective case series by Steen et al. there were three women with prior renal crisis who continued ACE inhibitors during pregnancy and each managed a successful pregnancy, and one had an abortion; Also there were two women with early diffuse scleroderma renal crisis that developed during pregnancy; one of them required dialysis temporarily and had a preterm baby, and the other had an elective abortion but required dialysis despite this.18
There is no evidence that elective abortion, when a renal crisis occurs, reverses the changes; however, in cases of great maternal or fetal health compromise, pregnancy termination may be considered, coupled with the initiation of therapy with ACE inhibitors.
The treatment of a renal crisis may require dialysis temporarily; there are cases of patients who required dialysis for up to 2 years after the renal crisis and eventually recovered enough renal function to stop dialysis.71
If presented with a worst case scenario during pregnancy, in which the physician is faced with a patient with rapid progression of skin involvement and/or rapid deterioration of organ function, the clinical decision can be guided by the weeks of gestation and the wishes of the patient. In the first quarter, termination of pregnancy should be considered and, during the third trimester, the induction of labor is an option in both cases in order to allow intensive treatment to decrease the rate of disease progression; if these options are not possible, at any time during pregnancy, the patient can be treated symptomatically with permitted drugs, trying to bring the patient to the point where she can undergo preterm labor and receive the same intensive care afterward.31
Obstetric surveillance. As previously discussed, there are certain most common complications in women with SS, especially when these are associated with APS or nephropathy, which are important determinants of perinatal outcome. Therefore, in these women, it is widely recommended to first determine the gestational age by performing an ultrasound during the first trimester of pregnancy; in particular, it is important to carry it out ultrasound at 11–13 weeks, 6 days, because it allows us not only to identify chromosomal abnormalities and some birth defects, but also to screen for placental disease by measuring the pulsatility index of uterine arteries and thereby detect which patients are at risk for preeclampsia and intrauterine growth retardation. According to a systematic review by Bujold,72 the use of aspirin at low doses during pregnancy, as long as one starts before week 16, can reduce by 52% the likelihood of preeclampsia when patients are at high risk for the disease, and considered appropriate in this group of patients, with low doses (80–160mg/day), from the end of the first quarter.
Obstetric Surveillance should include a structural ultrasound in the second quarter and also closer monitoring of fetal growth, with an ultrasound during the second half of pregnancy.
Management of LaborAt the time of delivery, the physician should consider that patients with SS can be a challenge for the anesthesiologist because of skin changes and the consequent difficulty for peripheral access, placement of a spinal block, achieving position during labor and monitoring with pulse oximeters and sphygmomanometers. In general, these patients are considered candidates for epidural block, which provides adequate anesthesia, peripheral vasodilation and increased skin perfusion.73–75 Some authors indicate lower dosage of anesthetic normally used because they may have longer postpartum sensory and motor block.74 General anesthesia is not recommended due to the difficulty for intubation and the risk of aspiration. It is recommended to monitor the room environment to prevent Raynaud's phenomenon associated complications. The measures used include: childbirth in a warm room, preheat IV fluids, use warm compresses, gloves, thermal socks, etc.
A central line or even a Swan-Ganz catheter may be required for central access, if cardiopulmonary complications, hypertension or renal failure are contemplated.
Depending on the fetal condition in late pregnancy, should the patient opt for a normal delivery, the fetus must be continuously monitored electronically.
Care of the surgical wound, cesarean or episiotomy, must be considered if there is skin involvement in the abdomen or vulva, but there is no published data on local complications and skin involvement in the abdomen has not been associated with the need to end the pregnancy prematurely.
Postpartum, the patient must be provided with continuous monitoring to address timely complications that may arise, remembering that heart failure and cardiovascular collapse in patients with PAH are the most lethal complications. Drugs that have been suspended during pregnancy should be restarted and the clinician should not wait to see if they will be required again.
Table 2 shows a summary of the recommendations for the management of pregnancy in patients with SS.
Recommendations for the Management of Pregnancy in Patients With SS.
| Before pregnancy or during the first trimesterEvaluation in the early phase of the disease, of the extent of organ involvement and antibody testing to establish potential risksDiscontinuing drugs with teratogenic potential preferably 3 months before pregnancy begins |
| Throughout pregnancyHigh-risk obstetric careUsing proton pump inhibitors, histamine receptor blockers and magaldrate in the smallest possible dose, for gastrointestinal symptomsMinimize the use of corticosteroidsIncrease the frequency of monitoring of fetal size and uterine contractionsFrequent monitoring of maternal BPIntensive treatment of hypertension according to the following general guidelinesAny further increase in the BP should be considered serious and require urgent evaluationMonitor if increased creatinine, proteinuria or microangiopathic hemolytic anemia, elevated liver enzymes or uric acidDo not assume it is preeclampsiaGive treatment needed to control BP with medications allowed during pregnancy, but if creatinine increases, add ACE inhibitors at low doses and increase them as needed, despite the risk of fetal toxicity. BP control can save the life of the mother and fetus |
| Labor and deliveryPrefer epidural anesthesiaAdequate temperature of the delivery room, patient and IV solutionsAntepartum placement of central venous accessSpecial Care performing an episiotomy or cesarean section |
| PostpartumMonitoring of vital signs the first daysReinstatement of drugs for the treatment of SS and intensive treatment of any hypertensive eventClose monitoring for possible heart failure, particularly in patients with heart failure and/or PAH, the first 2–4 weeks postpartumClose monitoring of renal function |
SS: systemic sclerosis; PAH: pulmonary arterial hypertension; ACE inhibitors, angiotensin converting enzyme inhibitors; BP: blood pressure.
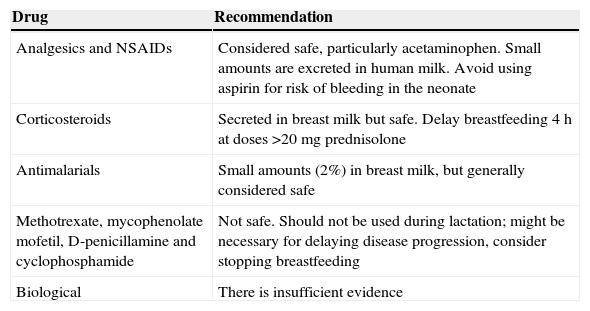
As already mentioned, once the pregnancy is completed, treatment should be reinstated for SS in all patients; however, breastfeeding should be taken into consideration, considering whether the drugs are safe, so this process should be individualized for each patient based on their desires and the need for drugs during this period.20 The general recommendations are listed in Table 3.
General Recommendations on the Use of Drugs During Puerperium and Lactation in Women With SS.
| Drug | Recommendation |
|---|---|
| Analgesics and NSAIDs | Considered safe, particularly acetaminophen. Small amounts are excreted in human milk. Avoid using aspirin for risk of bleeding in the neonate |
| Corticosteroids | Secreted in breast milk but safe. Delay breastfeeding 4h at doses >20mg prednisolone |
| Antimalarials | Small amounts (2%) in breast milk, but generally considered safe |
| Methotrexate, mycophenolate mofetil, D-penicillamine and cyclophosphamide | Not safe. Should not be used during lactation; might be necessary for delaying disease progression, consider stopping breastfeeding |
| Biological | There is insufficient evidence |
NSAIDs: Non-steroidal anti-inflammatory drugs; SS: systemic sclerosis.
- -
A large proportion of women with SS can lead successful pregnancies with little risk of serious complications if the patient and their doctors discuss the issue and choose a suitable time for pregnancy, and close obstetric monitoring is performed.
- -
Pregnancy in women with SS should be considered, from the onset, as a high risk pregnancy due to increased risk of premature delivery and low birth weight for gestational age.
- -
In early pregnancy, the clinician should carefully assess each patient to establish the subtype of disease being treated (diffuse or limited), the (early or late) phase and the extent and severity of damage to internal organs.
- -
Those patients with duration of symptoms less than 4 years (scleroderma in early stage), diffuse subtype, or anti-topoisomerase i or anti-RNA polymerase iii antibodies have higher associated obstetric risk and, if possible, should delay pregnancy until the patient is in a late stage and therefore has a less active disease.
- -
The third trimester of pregnancy is considered the one with the highest risk, since the patient may develop complications secondary to hypertension, renal failure, pulmonary hypertension, interstitial lung disease or heart failure.
- -
The consensus of many studies is that there are no other significant changes in the activity of the SS during pregnancy.
- -
The evidence indicates that patients with SS have no fertility problems resulting from their disease.
The authors declare that no experiments were performed on humans or animals for this article.
Confidentiality of dataThe authors state that no patient data appear in this article.
Right to privacy and informed consentThe authors state that no patient data appear in this article.
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Rueda de León Aguirre A, Ramírez Calvo JA, Rodríguez Reyna TS. Manejo integral de las pacientes con esclerosis sistémica durante el embarazo. Reumatol Clin. 2015;11:99–107.