Osteomalacia is a rare metabolic bone disorder which can be caused by various diseases associated with a deficiency of calcium, phosphorus or an inhibition of the mineralization process.1 We report the case of a 62-year patient presenting hypophosphatemic osteomalacia diagnosed through laboratory data, with no clear etiology, classified as idiopathic.
The patient exhibited bilateral hip fractures of a non traumatic origin and had received a prosthesis which had undergone mobilization. He presented muscle and bone pain lasting 3 years that limited movement. She reported no dentition or audiological changes, no prior medication or family history of kidney or bone disease.
Laboratory analysis showed: normal CBC and venous blood gases, renal and hepatic profiles were normal except for elevated alkaline phosphatase (443IU/L for n<136), normocalcemia and hypophosphatemia 0.60mmol/L (n=0.7–1.60) and elevated iPTH (79.8ng/L for n<67) with normal values of 1.25 OHD and 25(OH)2D. The 24-h urine had normal calcium and phosphate, with no other useful information. Hydroxyprolinuria of 41.4mg/L (<17/24h/m2).
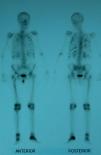
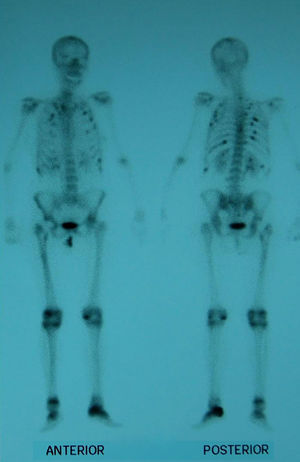
Suspecting hypophosphatemic osteomalacia we obtained the following test results in search of its etiology: normal serum protein on electrophoresis with absence of Bences Jones protein in the urine, normal tumor markers (carcinoembryonic antigen CEA, 15.3, 19.9, 125, alphaglycoprotein); we studied the patient for possible digestive malabsorption syndrome (endoscopic, X-ray study, histological and celiac antibodies) but results were negative; parathyroid study (neck ultrasound and sestamibi scintigraphy) was normal as were chest X-rays and abdominal ultrasound; a bone scan with Tc99 showed multiple uptake areas in the ribs, heel, and periprosthetically in both hips and peripheral joints (Fig. 1). Suspecting a possible oncogenic cause we performed an 11in.-octreotide scintigraphy for the detection of somatostatin receptors which resulted negative. Bone mineral density (BMD) of the lumbar spine (L2–L4) was 0.596g/m2 with a T score of −4.74. We did not study the levels of fibroblast growth factor (FGF-23).
We started treatment with calcitriol (0.75mcg/24h) and sodium phosphate (Phosphate-Sandoz®, 2g/24h) achieving normalization of blood phosphorus levels, iPTH and FA, with subsequent hyperphosphaturia in urine 24h (2538mg/L) which had not previously been observed. After this parameter normalized we added strontium ranelate treatment for the treatment of osteoporosis. The patient's symptoms gradually improved. Three years after the diagnosis, after initiating treatment, the patient can walk, mineral metabolism analytical controls are normal as well as the Tc99 bone scintigraphy. The latest lumbar spine densitometry (L2–L4) shows 0.744g/m2, with a T score of −2.93 and a −0.82 Z score.
The parameters studied suggest that this case corresponds to osteomalacia characterized by insufficient mineralization of the osteoid formation in new bone metabolism points. Different diseases can lead to osteomalacia through mechanisms that cause hypocalcemia, hypophosphatemia or defects in the mineralization process.1 In this case, the slight decrease in phosphoremia suggests that it is hypophosphatemic osteomalacia. Interestingly, phosphoremia was initially accompanied with normality of phosphaturia probably due to a severe depletion of phosphate reserves, responsible in turn of the evolved osteomalacia process with atraumatic fractures and crippling myopathy.
The case of the patient under study is remarkable because it was not associated with any of the causes described in hypophosphatemic osteomalacia1–4: (i) no family history or other evidence of heredity (e.g. abnormal dentition), (ii) no deficit of phosphate in the diet, (iii) or an association with any drug, (iv) no abnormalities were found that made us suspect a parathyroid endocrine origin; (v) no digestive malabsorption disorders or (vi) kidney problems; (vii) finally, the normality of 11in.-octreotide scintigraphy, tumor markers, Bences Jones proteins in the urine, and abdominal ultrasonography Chest X-rays suggest an unlikely oncogenic source.
We could not, to date, find the mechanism that caused the disturbance, which require us to classify it as idiopathic and keep looking for new possible causes of this disease. Known cases of idiopathic hypophosphatemic osteomalacia are rare.5,6 However, treatment based on phosphate supplementation has been effective in stabilizing blood phosphorus levels, but leads to a mild phosphaturia, to be expected based on the contribution.
Conflict of InterestThe authors have no disclosures to make.
Please, cite this article as: León Rubio P, Baturone Castillo M. Osteomalacia hipofosfatémica idiopática. Reumatol Clin. 2013;9:327–328.