The appearance in the field of oncology of therapeutic molecules in the form of monoclonal antibodies, whose objective is to stimulate the patient's own immune system to be responsible for destroying cancer cells, has revolutionized the treatment of many cancers in recent years. This type of therapy, called immunotherapy, is also characterized by presenting side effects in the form of autoimmune diseases that we are still beginning to understand. From the point of view of the immune-mediated rheumatological side effects, we can find musculoskeletal manifestations, mechanical, inflammatory or systemic autoimmune diseases. The therapeutic approach to these side effects remains uncertain due to the absence of clinical trials and validated recommendations. The multidisciplinary management is crucial to successfully treat such cases. In the following manuscript, we will describe our case reports of rheumatologic immune-related adverse events in a university hospital.
La aparición en el campo de la oncología de moléculas terapéuticas en forma de anticuerpos monoclonales, cuyo objetivo consiste en estimular el propio sistema inmune del paciente para que sea este el encargado de destruir las células cancerígenas, ha revolucionado el tratamiento de diversos cánceres en los últimos años. Este tipo de terapia, denominada inmunoterapia, se caracteriza además por presentar efectos secundarios en forma de enfermedades autoinmunes que todavía estamos empezando a conocer. Desde el punto de vista de los efectos secundarios inmunomediados reumatológicos, podemos encontrar manifestaciones musculoesqueléticas mecánicas, inflamatorias o enfermedad autoinmune sistémica. El manejo terapéutico de estos efectos secundarios se mantiene variable debido a la ausencia de ensayos clínicos y de recomendaciones validadas, siendo el manejo multidisciplinar fundamental para tratar con éxito dichos casos. En este artículo presentamos nuestra serie de casos clínicos de pacientes en tratamiento con inmunoterapia y efectos secundarios inmunomediados reumatológicos en un hospital universitario.
Immune checkpoint inhibitors against cytotoxic T-lymphocyte-associated protein 4 and programmed cell death protein 1 and its ligand have significantly improved survival in a great many varieties of cancers. However, immunotherapy is responsible for the so-called immune-related adverse effects (irAE) which are produced by several mechanisms of action.1
In this article we describe our experience in clinical practice in patients who developed rheumatologic IrAE secondary to immunotherapy.
Description of the clinical case seriesA retrospective review was made of patients treated with immunotherapy (n=170) in the Complejo Hospitalario Universitario Insular Materno Infantil from 2014 to 2017. Patients who developed rheumatologic symptoms after initiation of immunotherapy were included. These patients were assessed by the Rheumatology Department to confirm or rule out any underlying rheumatic disease. Out of a total of 170 patients who received immunotherapy, 16 (9.4%) presented with rheumatologic symptoms. Two patients with lumbago were excluded because this was associated with bone metastasis. The mean age of the remaining 14 patients was 59.1 years (46–80 years). 64.2% were men. The mean time of musculoskeletal symptom onset was 21.21 weeks (1–71 weeks).
Among the rheumatic symptoms presented by our patents, 9 developed arthromyaligas with no evidence of inflammatory disease; 4 developed inflammatory joint disease (olecranion bursistis, 2 inflammatory arthritis associated with the immunotherapy and one microcrystalline arthritis) and one patient developed a leukocytoclastic vasculitis.
Distribution according to type of tumour was as follows: metastatic melanoma (n=3), epidermoid carcinoma of the lung (n=3), adenocarcinoma of the lung (n=4), adenosquamous carcinoma of the lung (n=1), renal clear cell carcinoma (n=1), tracheal squamous carcinoma (n=1) and neuroendocrine carcinoma of the lung (n=1).
The immunotherapy used was nivolimumab in 11 patients, pembrolizumab in 2 patients and atezolizumab in one patient.
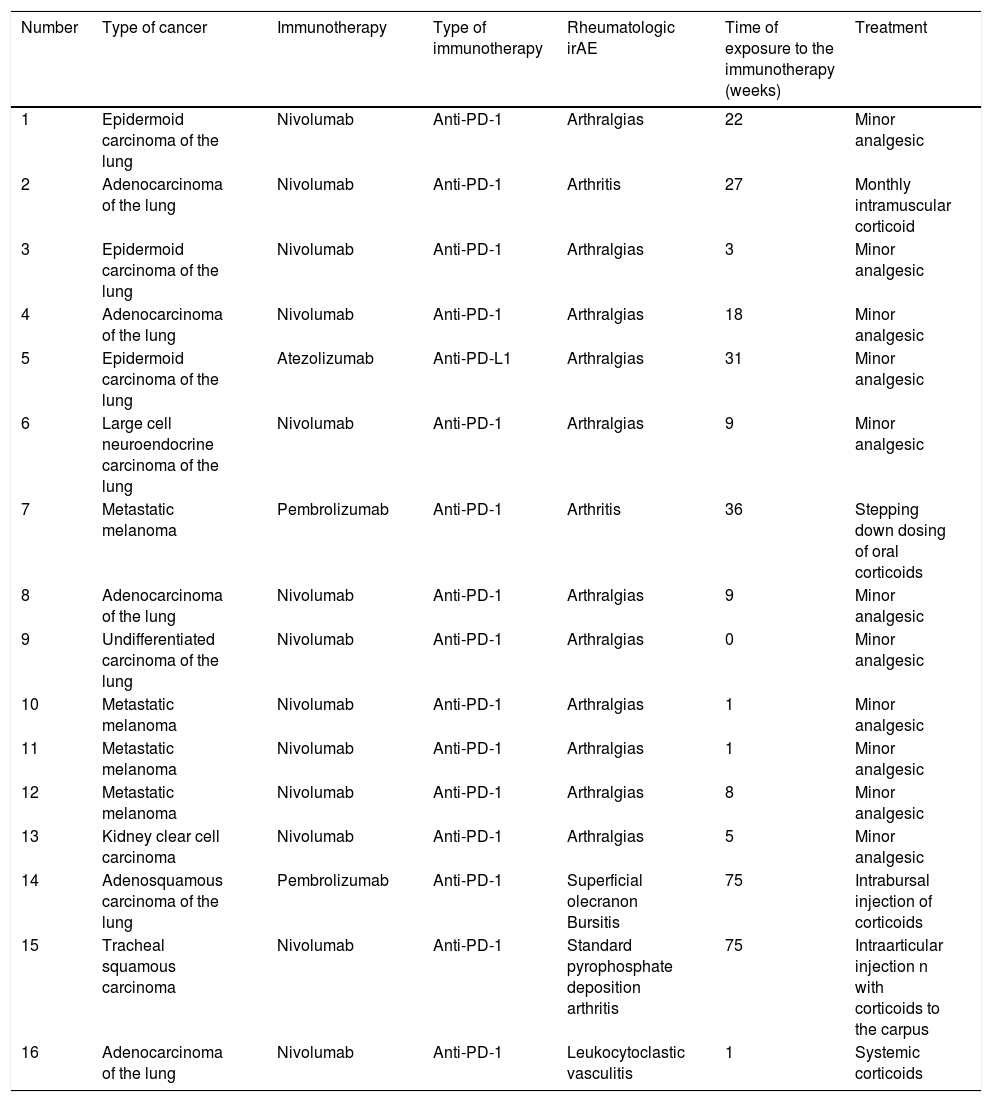
Demographic, clinical and treatment data are summarized in Table 1.
Demographic, clinical and treatment data.
| Number | Type of cancer | Immunotherapy | Type of immunotherapy | Rheumatologic irAE | Time of exposure to the immunotherapy (weeks) | Treatment |
|---|---|---|---|---|---|---|
| 1 | Epidermoid carcinoma of the lung | Nivolumab | Anti-PD-1 | Arthralgias | 22 | Minor analgesic |
| 2 | Adenocarcinoma of the lung | Nivolumab | Anti-PD-1 | Arthritis | 27 | Monthly intramuscular corticoid |
| 3 | Epidermoid carcinoma of the lung | Nivolumab | Anti-PD-1 | Arthralgias | 3 | Minor analgesic |
| 4 | Adenocarcinoma of the lung | Nivolumab | Anti-PD-1 | Arthralgias | 18 | Minor analgesic |
| 5 | Epidermoid carcinoma of the lung | Atezolizumab | Anti-PD-L1 | Arthralgias | 31 | Minor analgesic |
| 6 | Large cell neuroendocrine carcinoma of the lung | Nivolumab | Anti-PD-1 | Arthralgias | 9 | Minor analgesic |
| 7 | Metastatic melanoma | Pembrolizumab | Anti-PD-1 | Arthritis | 36 | Stepping down dosing of oral corticoids |
| 8 | Adenocarcinoma of the lung | Nivolumab | Anti-PD-1 | Arthralgias | 9 | Minor analgesic |
| 9 | Undifferentiated carcinoma of the lung | Nivolumab | Anti-PD-1 | Arthralgias | 0 | Minor analgesic |
| 10 | Metastatic melanoma | Nivolumab | Anti-PD-1 | Arthralgias | 1 | Minor analgesic |
| 11 | Metastatic melanoma | Nivolumab | Anti-PD-1 | Arthralgias | 1 | Minor analgesic |
| 12 | Metastatic melanoma | Nivolumab | Anti-PD-1 | Arthralgias | 8 | Minor analgesic |
| 13 | Kidney clear cell carcinoma | Nivolumab | Anti-PD-1 | Arthralgias | 5 | Minor analgesic |
| 14 | Adenosquamous carcinoma of the lung | Pembrolizumab | Anti-PD-1 | Superficial olecranon Bursitis | 75 | Intrabursal injection of corticoids |
| 15 | Tracheal squamous carcinoma | Nivolumab | Anti-PD-1 | Standard pyrophosphate deposition arthritis | 75 | Intraarticular injection n with corticoids to the carpus |
| 16 | Adenocarcinoma of the lung | Nivolumab | Anti-PD-1 | Leukocytoclastic vasculitis | 1 | Systemic corticoids |
irAE: immune-related adverse effects; PD-1: programmed cell death protein 1; PD-L1: programmed cell death ligand-1.
The different mechanisms of action of the immunotherapeutic agents is reflected in the different side effects they induce. Cytotoxic T lymphocyte protein 4 inhibitors tend to produce more frequent and severe adverse effects (colitis type gastrointestinal conditions, cutaneous and hypophysitis type endocrine conditions), whilst the programmed cell death protein 1 and its ligand inhibitors are more commonly associated with systemic autoimmune diseases, pneumonitis or thyroiditis.2 Combined or double block therapy leads to greater improvement in clinical response compared with monotherapy, and also an increased risk of IrAE.1,2
IrAE usually appear from the first few weeks of treatment up until the first 3–6 months of its administration.1
A systematic review of the literatura3 proves that the most commonly observed rheumatic IrAE in patients with immunotherapies are arthralgias (1%–43%), myalgias (2%–21%), rheumatoid athritis, psoriasic arthritis, RS3PE (1%–7%) syndrome and sicca syndrome (3%–24%).
During the last few years several clinical series have been published which have described rheumatic irAE in clinical practice.3–6
There are recommendations for handling the different rheumatologic irAE created by the Toxicity Work Group of the Cancer Immunotherapy Society7 and other work groups. In general, the diagnostic algorithm is similar to that of a patient with a rheumatologic disease, whilst the treatment that is usually administered uses higher corticoid guidelines than those recommended, and DMARDD type immunosuppressants (both synthetic and biologic). On rare occasions immunotherapy needs to be discontinued.7
The use of immunosuppressant treatment or biologic therapy for treating IrAE is controversial, since it could increase the risk of developing IrAE8 or interfering in the efficacy of the immunotherapy.9–11 There are retrospective studies which show that survival does not differ between patients who were treated with immunosuppression and those who did not received it.9
Immunotherapy is broadening the horizons of the area of rheumatology. This is possibly unconventional but also exciting. A multidisciplinary approach would help to improve the quality of patient care.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Nóvoa Medina FJ, Tejera Segura B, González Rodríguez E, Machín García S, Romero Díaz B, Rodríguez Abreu D. Inmunoterapia, cáncer y enfermedades reumatológicas: revisión de la literatura y serie de casos en un hospital universitario. Reumatol Clin. 2020;16:413–415.