Fibrodysplasia ossificans progressiva is the most severe and disabling disorder of ectopic ossification in humans. It is characterized by congenital skeletal abnormalities in association with extraskeletal widespread endochondral osteogenesis. Virtually all patients show the same mutation in the “activin A type-I/activin-like kinase-2” receptor encoding gene. As a result of this discovery there have been significant advances in the knowledge of the cellular and molecular basis of the disease. Besides allowing a better understanding of ossification process, recent evidence indicates that the primary disturbance lies within basic mechanisms of cell differentiation that are key in several physiological pathways and in the genesis of diseases with a major impact on health. In this article we summarize these breakthroughs, with implications that go beyond the limits of this devastating disease to insinuate a new model of human pathophysiology.
La fibrodisplasia osificante progresiva es la causa más grave de osificación ectópica en humanos. Se caracteriza por malformaciones esqueléticas congénitas y placas de hueso maduro (endocondral) en el músculo y en otras estructuras ricas en tejido conjuntivo. Se produce por una mutación espontánea en el gen del receptor de la activina A tipo i, similar a la activina-cinasa-2. A raíz de este hallazgo, se han producido importantes avances en el conocimiento de su base molecular y celular. Además de permitir una mejor comprensión de los mecanismos que gobiernan la osificación, evidencias recientes indican que la alteración primordial radica en mecanismos básicos de la diferenciación celular que son clave en varias vías fisiológicas y en la génesis de enfermedades de gran impacto. En el presente artículo, resumimos los últimos avances con implicaciones que trascienden los límites de esta devastadora enfermedad para postularse como un nuevo modelo dentro de la fisiopatología humana.
Fibrodysplasia ossificans progressiva (FOP, MIM #135100) is one of the most unique constitutional bone diseases, to the point of being considered “the Mount Everest of musculoskeletal disorders of genetic origin”1 and a model applicable to research in regenerative medicine and in the knowledge of the metamorphosis of tissue.2
Main Epidemiological FeaturesThe scarcity of epidemiological data is a problem that FOP share with all rare diseases and, in a certain way, with most other low prevalence musculoskeletal diseases.3 Scattering processes inherent to these cases and their complexity, with the added diagnostic difficulty involved, makes it very difficult to conduct population studies due to the lack of a reliable census of cases with a definitive diagnosis.4 However, a fact that is accepted as universally valid, despite being an estimate obtained by extrapolation from the results of a single pioneering study, is the overall prevalence: about one case per 2 million inhabitants.5 This seems consistent across countries and geographical areas where comparable studies have been conducted, so it is considered that there is no clear ethnic or gender predisposition.6–8 Similarly, a study conducted in Spain identified 17 patients who survived to the end of 2011 (point prevalence=0.36×10−6) and 24 were included in the total sample, consistent with the estimated frequency other areas.9 Moreover, as discussed in detail below, the majority of cases occur de novo or as the result of a spontaneous mutation.6,7 Thus, the familial aggregation of FOP is extremely rare and has been reported in only a very small number of families with few cases in 2 generations.6 At different times and in different geographical areas there have been reports of a greater predisposition linked to paternal age5,9,10 that could be related to the known universal effect of advanced paternal age as a factor favoring mutations. Additionally, exposure to some potentially mutation inducing environmental agents has been reported.9 However, the difficulties and inherent methodological shortcomings of these studies (where it is very difficult to establish appropriate controls and virtually impossible to investigate the existence of a dose–response effect) require very careful interpretation of these inferences before assigning a causal value.
Clinical Diagnosis and Current Treatment OptionsFrom the clinical point of view, FOP is characterized by congenital malformations and the development of mature bone plates (with a pattern of endochondral ossification) within the muscle and other connective tissue rich structures.11 The virulence of these alterations places FOP as the most serious cause of ectopic ossification in humans.12
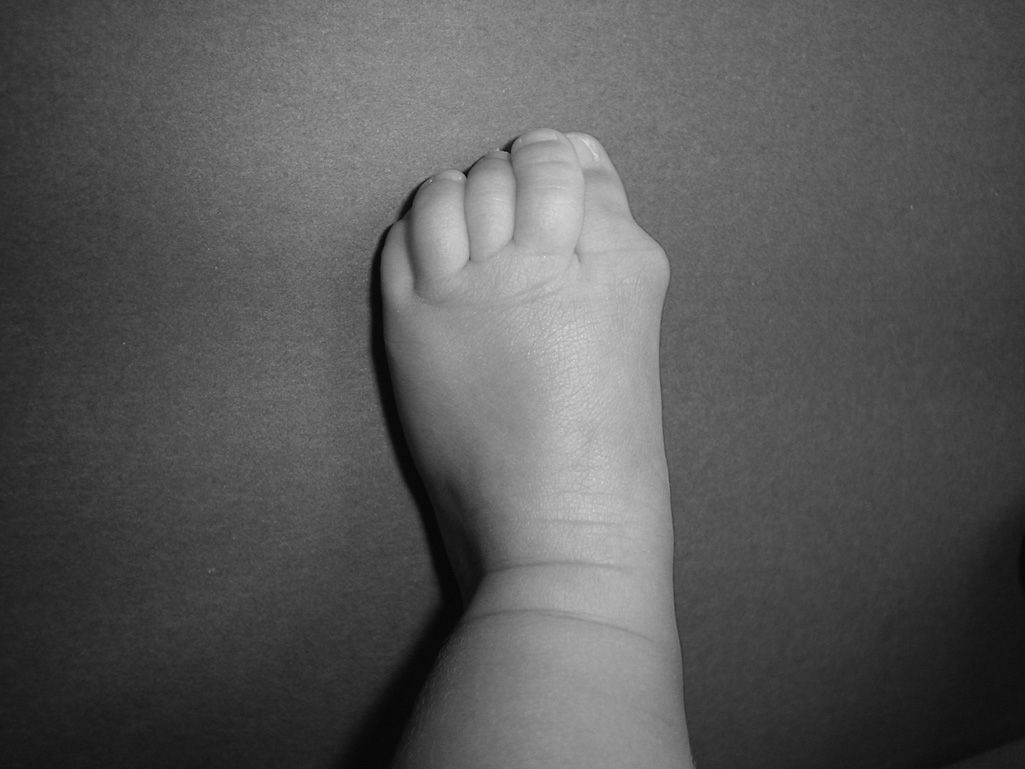
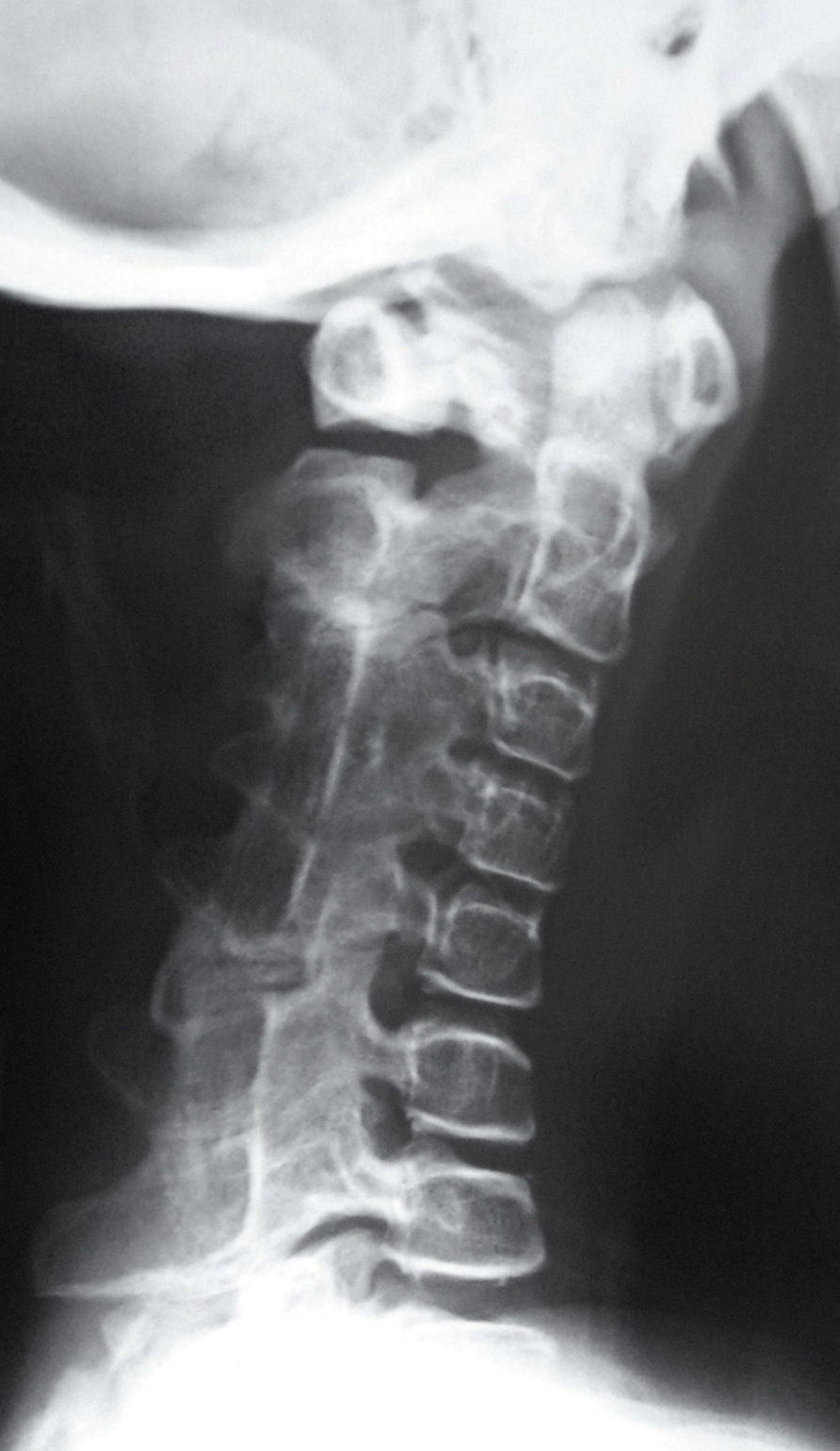
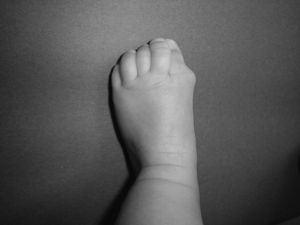
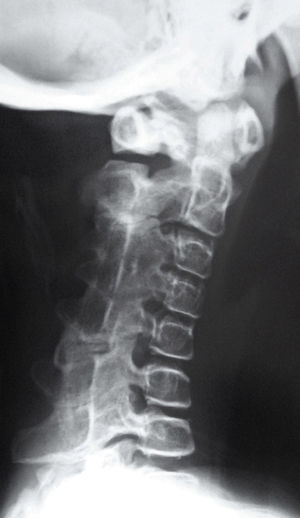
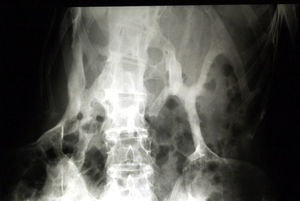
Newborns with FOP appear normal except for the presence (in almost all of them) of malformations in the first toe, with congenital hallux valgus being the hallmark of the disease (Fig. 1).13,14 Although usually recognized later (and even unnoticed or misinterpreted), the dysplastic component of FOP can be manifested as various congenital skeletal abnormalities that appear with varying frequency but which is almost always high. These malformations include: other abnormalities in fingers and affection of other toes (shortening of phalanges, metacarpals and metatarsals, synostosis, clinodactyly)15; increased size and subsequent fusion of the vertebral facets with hypoplasia of the vertebral body in the cervical spine (Fig. 2) which may end up forming a block16–18; osteochondromas, especially evident in the medial aspect of the tibia,19,20 and short and wide femoral neck.
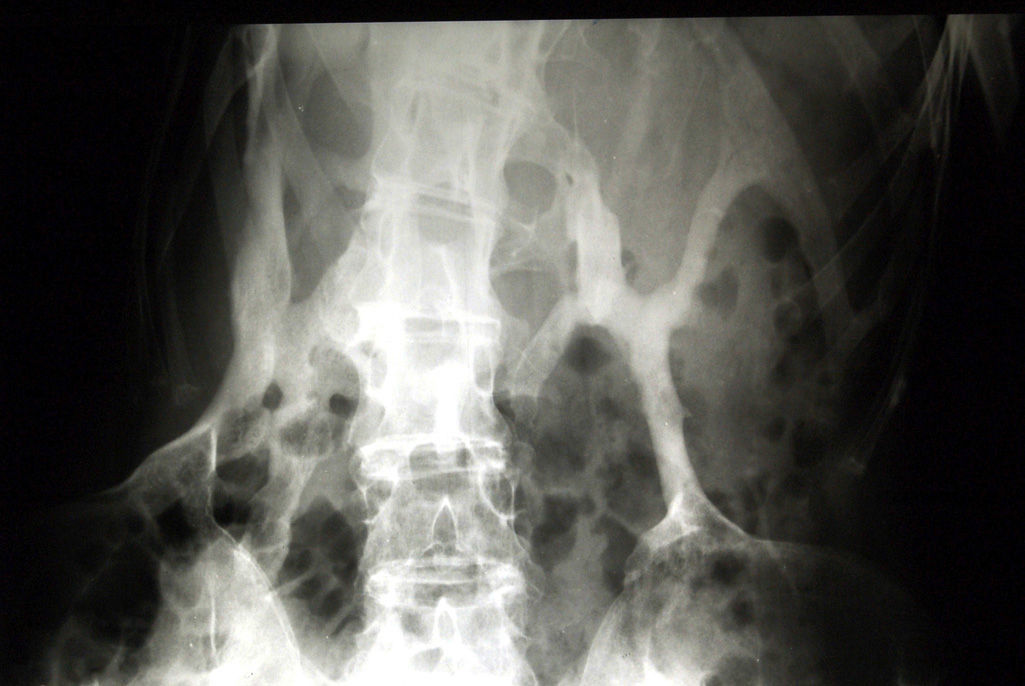
Moreover, there is some variability in terms of onset and intensity, from the first months or years of life, often triggered by trauma, and most patients present acute episodes of formation of pseudoinflammatory nodules.2,7,12 Over time, these lesions present within striated muscle, and tendons and ligaments are transformed into mature bone Plates11 which extend and progress sequentially according to a specific anatomical pattern: cephalic–caudal, proximal–distal and axial–appendicular.21 Thus, although the rate of progression is variable, between the second and third decades of life, the plates start branching forming a “second skeleton”1,22 with rigid bridges that decrease the mobility of the structures upon seating (Fig. 3).7,12 As the plates are usually located in anatomical regions of high functional importance such as the neck, shoulders, hips and knees, it almost always ends up compromising mobility and basic activities such as walking. Later, they can affect vital regions such as the submandibular area–giving rise to trouble chewing, swallowing and talking–and chest muscles, which makes breathing difficult and causes serious complications that can lead to an early death.12,23 In addition, FOP can cause other disorders which affect essential functions, such as hearing loss, present in a high percentage of patients.24 Recently, a study based on a postal questionnaire sent to a large sample of patients reported a high frequency of chronic neurological symptoms.25 Along the same line (and aware that the findings require confirmation), two animal models and imaging techniques have observed demyelinating lesions in the central26 nervous system, suggesting that this could be related to the deregulation of the signal pathway of bone morphogenetic proteins, as will be seen later, which play a key role in the pathogenesis of FOP.
Increasingly, more attention is being paid to the heterogeneity in the clinical expression of FOP,27,28 which seems to depend on the differential influence of genetic and environmental factors.29,30 In 3 pairs of monozygotic twins it was found that each pair of brothers had malformation of the first toe, being the same and different from those of the other31 pairs. Based on this evidence and observations on the clinical course of the disease, these authors suggest that genetic factors would condition a disturbed fetal development, while the lifestyle associated environmental agents (repeated trauma is the best know factor) partly determine the intensity and pace of ossification.
In the early stages, before the formation of heterotopic bone plates, diagnostic errors are common, confounded by processes such as aggressive juvenile fibromatosis, lymphedema or soft tissue sarcomas.12,32,33 However, it is very important to note that although the incipient nodules can be misleading, the concomitant presence of a typical congenital malformation of the first toe (Fig. 1) allows an accurate diagnosis. In any event, the appropriate genetic test confirms the disease, making other tests unnecessary and allowing therapeutic measures may result in irreversible damage.34 In this regard, we must insist on the futility of the biopsy, which, like orthopedic surgical procedures, should be avoided, as it routinely leads to worsening of the injury.33–35
Currently, there are no effective treatments for FOP. Consequently, efforts are directed at the prevention and symptomatic relief of outbreaks of nodules (before the formation of bony plates), supportive and functional recovery, aids,23,33 and genetic counseling. It is very important to avoid or minimize any factors that may trigger or aggravate the development of plaques, including trauma, intramuscular injections and any other aggressive intervention that may affect the integrity of the connective tissues. Also the clinician should exercise extreme care in avoiding dental caries, recommending that their treatment be performed by specialists familiar with complex situations.36 Also, if there is a need for general anesthesia (any surgical indication should be justified), the anesthesiologist should consider the risk of an atlanto-axial dislocation occurring and master techniques for special intubation to minimize this complication.37,38 For a detailed description of the patterns of symptomatic outbreaks of initial nodes and attempt to prevent their conversion into bone plates through treatment with corticosteroids, reading the guidelines and recommendations published by the “International Clinical Consortium for FOP” is indicated.33 For the purposes of this review, we conclude here this first part and move on to analyze the findings provided by the latest genetic research and biochemistry, which seem to open a door to the achievement of a treatment that effectively alters the natural history of this39,40 devastating disease.
FOP Mutation and Bone Morphogenetic ProteinAlmost all patients that show the classic FOP phenotype described above have an identical–c.617 G>A–mutation in the gene encoding for activin receptor type I like the activin-kinase-2 (ACVR1/ALK2), which is heterozygous and antisense, recurring at codon 206 (R206H) within the GS domain of the receptor mutation.41,42 The ACVR1 receptor/ALK2 is expressed in tissues such as skeletal muscle, blood vessels (endothelial cells and pericytes) and cartilage, among others, which would explain the alterations in skeletal development and the characteristic heterotopic ossification of the “FOP phenotype”.41,43,44
The ACVR1/ALK2 transmembrane receptor belongs to a family of receptors for bone morphogenetic protein (BMP), which are considered members of the superfamily of transforming growth factor-beta (TGF-β).45 Conservation throughout evolution and its presence in all multicellular organisms highlight the importance these cytokines exert on their effects in multiple tissues and systems. The TGF-β/BMP receptor superfamily shows a very complex and schematically heterotetrameric structure, and may fall into 2 categories46:
- -
Type I includes 7 receptors (ALK1–7) with the common characteristic of being “activin kinase receptor”. ACVR1/ALK2 belong to this type, whose mutation is present in FOP.
- -
Type II: this has been described in up to 5 different receptors.
A peculiar characteristic of all TGF-β/BMP type ireceptors is a region rich in glycine and serine residues juxtaposed on the cytoplasmic membrane: the GS domain.47–53 many findings indicate that this area is critical in the signaling and, after ligand binding, triggering of at least two cascades of signal transmission. In the case of ACVR1/ALK2 receptors, interaction with BMP ligands (especially BMP-2, 4, 6 and 7) leads to the association of the type I receptor with the type II receptor that is constitutively active and, in turn, phosphorylates the type I receptor on the GS residue. This modification causes the displacement of the inhibitory protein FKBP1254 and subsequent signal transduction through: (a) a canonical pathway, characterized by activation of the R-Smads1/5/8 proteins. Phosphorylation of these factors allows coupling with the Co-Smad (Smad4) and its translocation to the nucleus, where it directs the transcription of specific genes, many of them directly involved in the establishment of a proosteogenic environment, and (b) a non-canonical pathway, characterized by signaling through the “mitogen activated protein kinase” (MAPK), which includes p38 enzymes, “extracellular signal-regulated kinase” (ERK) and “terminal kinase of c-jun” (JNK). Unlike the canonical pathway, the regulation of these kinases by BMP/TGF-β ligands has not been studied in depth. However, the fact that this cascade of “secondary” signaling is not exclusive of the TGF-β/BMP pathway indicates that its control could play an integral role in other signal transduction cascades (inflammatory pathways,55 Wnt56 and Notch57 ligands), which also activate MAPK.
In summary, this set of facts suggest that the ligands of the “TGF-β family’ occupy a central position in the network of signals that govern the growth, differentiation and fate of progenitor cells in a variety of cells and tissues, both during embryonic development and after birth.58–60 In particular, due to the key role of BMPs in embryogenesis and tissue homeostasis in the adult organism, their alterations have been implicated in the pathogenesis of several diseases of a diverse nature.61
The FOP Metamorphogene: Deregulation of the “BMP Pathway” SignalIn vitro studies of cell models have uncovered that the canonical FOP mutation induces quasi constitutive activation of ACVR1/ALK2 receptors that causes an interaction between the amplifier and one of the key enzymes in the process of intracellular signal transduction, the peptidyl prolyl cis–trans isomerase (FKBP1A/FKBP12), thus increasing the activation of several proteins BMP pathway43,62–64 without44 ligand–receptor interaction. Simple substitution of an amino acid (arginine and histidine), leading canonical FOP mutations, transforms a metamorphogenetic receptor into a morphogenetic receptor, providing a substrate which makes developing ante and postnatal disease changes possible.58
Consistent with this premise, various experimental models have shown that the constitutive activation of the mutated receptor: (a) induces alkaline phosphatase activity in muscle stem cells (Satellite cells) of mice (C2C12),63,65 (b) upregulates BMP-4 and low cascade antagonists of the BMP signal through the Smad pathway (see below),44 (c) joint elements expand through the induction of ectopic chondrogenesis and joints fuse, similarly to what happens in FOP,43,59,65 and (d) regulates the stability of the messenger RNA of ACVR1/ALK2, acting as a positive reinforcement loop.66
Moreover, the results obtained in Drosophila melanogaster, in which the regulation of the “Dpp pathway” (BMP homologous gene) in “decapentaplegic” mutants,67 was studied, as well as the phenotypic analysis of42 patients, indicate that the pathogenic alteration affects the embryonic modeling as well as abnormal postnatal responses to stimuli capable of causing tissue injury.58 Some of the most important findings supporting this theory are increased BMP expression in cells of active lesions62,68,69 and an increase in the concentration of BMP type I receptors on the cell surface.70 An increase in osteogenic differentiation of connective tissue progenitor cells obtained by exfoliation of teeth has also been reported: “SHED” cells (“stem cells from human exfoliated decidous teeth”). In healthy volunteers, these cells transmit both BMP signaling through the Smad pathway and MAPK pathway (in particular through p38 kinase) and respond to treatment with BMP-4. In FOP patients, they show increased baseline and after ligand stimulation responses and differentiate more rapidly than those of the controls in an osteogenic phenotype.71
The difficulty and risk inherent in obtaining primary tissues from patients with FOP35 have stimulated the development of in vivo models. Therefore, thanks to experiments in a zebrafish model that expresses the mutant receptor, it has been shown that this gene induced chondrogenesis independently of ligand.43 Soon after, it was confirmed that the expression of the mutated human receptor in FOP, R206H ALK2 in D. melanogaster caused overactivation of the BMP signaling pathway. Interestingly, these experiments demonstrated that the mutated ACVR1/ALK2 receptor requires a functional BMP receptor type II.72 Recently, these findings were confirmed in a FOP mouse model, which consists of the expression of a ALK2 constitutive receptor with a73 Q207D mutation, opening the possibilities of another therapeutic target for drug development. However, in vitro studies have shown functional differences between the ALK2 R206H receptor and the Q207D receptor.64 At present it is not possible to have a mammalian model (preferably mice or rats) to simulate the full human FOP phenotype because the mutation is lethal during prenatal development in these animals. Perhaps the most significant advance in this direction came in 2012 with the publication of an ALK2 R206H chimeric mice model in which the expression of the mutated gene is carried out at a later stage of prenatal development, thus avoiding the lethality74 gene. However, this model is laborious and expensive, making it difficult to use to investigate the molecular mechanisms responsible for the disease, and the development of new drugs. In this respect, researchers have recently turned to the generation of stem cells obtained from primary tissue from patients.75,76 This strategy is intended to have unlimited material from patients in which new compounds that interfere with disease progression can be identified.
Key Mechanisms “Endothelial–mesenchymal Transition” and InflammationBoth epithelial plasticity and endothelial plasticity are essential for embryonic development and progression of certain diseases.77 The loss of endothelial features and the acquisition of mesenchymal characteristics–endothelial–mesenchymal transition (EndMT)– occurs in several biological processes of great importance, all related to alterations of the BMP. Thus, it has been implicated in cancer progression,77–79 in cardiac and renal fibrosis,80–83 in arteriosclerosis84–87 pulmonary hypertension and in the process of wound healing.88
The observation that chondrocytes and osteoblasts present specific endothelial markers in the repair of bone fractures stained with antibodies has led to the hypothesis89 that EndMT contributes to physiological repair mechanisms and may be involved in certain bone disorders. In this regard, in FOP patients and in animal models that mimic the disease, it has been shown that EndMT is an essential factor in the pathogenesis. Thus two transgenic mouse models (“NSE-BMP-4” and “MyoDicre”), designed to allow tracing of the origin of the cells responsible for skeletogenesis, it has been observed that, in an inflammatory microenvironment and response signals of the BMP pathway activated by the ACVR1/ALK2 mutation, endothelial progenitor cells differentiate into a chondrocyte line and contribute to each and every one of the stages of heterotopic ossification, from mesenchymal to endochondral bone formation.90 Before, with a different methods, evidence indicated that at least part of the mesenchymal cells involved in skeletal metamorphosis involved in FOP are of vascular origin.91 Recently, both patient samples obtained by biopsy and from animal models induced by the transfer of a ACVR1/ALK2 gene constitutively activated74 have shown immunohistochemically chondrocytes and osteoblasts present in the lesions expressing markers of endothelial vascular factors, such as Tie-2 and von Willebrand factor.92 Additionally, endothelial cells express mutant receptor and receptor ligands such as TGF-β-2 or BMP-4 and cause acquisition of EndMT similar to the mesenchymal stem cell phenotype.
Besides being influenced by stimulation of other signaling pathways, FOP is dependent upon the EndMT ligands TGF-β and BMP-β and mutated receptor ALK2 R206H. Therefore, chemical inhibitors of TGF-β (SB-431542 and LY-36494779) and BMP (based on dorsomorphin93–95 and with a different structures96) block EndMT. Moreover, due to the lack of specificity of the compounds developed so far because of the structural similarity of the ALK receptors, alternatives have been devised based on gene therapy to block the ALK2 receptor without affecting other receptors, thus avoiding possible side effects.97,98
Chondrogenesis requires99 an antiangiogenic hypoxic microenvironment. Because hypoxia is a consequence of inflammation,100 and because of its activation (particularly an due to an increase in prostaglandin E2 and proinflammatory cytokines such as tumor necrosis factor alpha101) identified as necessary to initiate heterotopic ossification,102 the interaction of both factors, together with other mechanisms, can be decisive in EndMT mediated90,94,102,103 R206H mutation. Moreover, it is well known that the stem mesenchymal cells capable of differentiating into adipocytes, osteocytes and chondrocytes, can interact with cells of the innate and adaptive immune system modulating various functions.104 While certain immune system cells derived from hematopoietic precursors have been implicated in skeletal metamorphosis characterizing FOP,41 their possible pathogenic role has still not been defined.
In summary, the predisposition to develop the endochondral heterotopic bone formation characteristic of FOP is caused by mutation R206H of the ACVR1/ALK2 receptor, which causes an upregulation of the signaling cascade of BMPs. This would be helped by microenvironment hypoxia associated with inflammatory activation occurring after trauma. This combination of factors would induce an “endothelial–mesenchymal transition” of vascular endothelial precursor cells, resulting in mesenchymal stem cells able to differentiate into osteoblasts and chondrocytes. Thus, endothelial cells participate in all stages of the formation of heterotopic bone (accumulation of mesenchymal cells, formation of osteoblasts and chondrocytes and bone maturation), focusing on anatomical regions where the ACVR1/ALK2 receptor is more expressed (skeletal muscle, blood vessels and cartilage). Thorough understanding of the mutations associated with FOP, the signaling pathway of BMP and mechanisms of “endothelial–mesenchymal transition” not only provide new therapeutic targets for drugs effective against this disease, but are a welcome development in the knowledge of a variety of basic processes in tissue and organ metamorphosis and repair.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that this study did not perform experiments on humans or animals.
Data confidentialityThe authors declare that patient data do not appear in this article.
Right to privacy and informed consentThe authors declare that patient data do not appear in this article.
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Morales-Piga A, Bachiller-Corral FJ, Sánchez-Duffhues G. ¿Es la «fibrodisplasia osificante progresiva» una enfermedad de origen vascular? Un modelo patogénico innovador. Reumatol Clin. 2014;10:389–395.