To assess the association between obesity, control of inflammatory activity and increased adverse effects in psoriatic arthritis (PsA) with disease-modifying anti-inflammatory drugs (DMARD).
MethodsA systematic literature review was performed using MEDLINE and EMBASE databases following the guidelines of the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) consensus statement. Studies were selected if they included patients with PsA, obesity was studied as a predictive factor, and the outcome was adverse effects, including efficacy failure. Quality was assessed using an ad hoc risk of bias tool. A qualitative analysis was carried out by type of study and study population, quality and specific results.
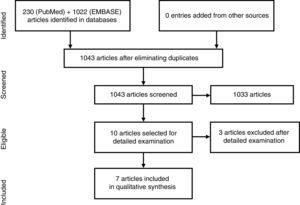
ResultsWe found 1043 articles, discarding most of them on the basis of title and abstract. Ten articles were studied in detail and finally excluded three. The majority concluded, with statistically significant results, that in patients with PsA and treated with TNFα inhibitors (TNFαi), obesity is associated with poorer chances of achieving and maintaining a minimal disease activity, higher treatment discontinuation rates, and lower skin response. Regarding conventional synthetic DMARD, a trend toward a moderate increase in transaminases with methotrexate (MTX) was observed in obese patients with PsA.
ConclusionsObesity is a negative predictor of clinical response in patients with PsA being treated with TNFαi. Except MTX hepatotoxicity, no other adverse effects, either with TNFαi or other drugs, were found in relation to obesity in PsA.
Estudiar si en la artritis psoriásica (APs) hay asociación entre la obesidad, el control de la actividad inflamatoria y el aumento de efectos adversos con los fármacos modificadores de la enfermedad (FAME).
MétodosRevisión sistemática de la literatura utilizando las bases de datos Medline y Embase según las guías del consenso MOOSE. Se incluyeron estudios en pacientes con APs, en los que la obesidad fuera factor predictor de efectos adversos y el desenlace fuera toxicidad, incluido fallo de eficacia. La calidad se evaluó mediante una escala de riesgo de sesgos ad hoc. Se realizó un análisis cualitativo por tipo de estudio y población estudiada, calidad y resultados específicos.
ResultadosSe encontraron 1.043 artículos, la mayoría se descartaron por título y abstract. Se estudiaron en detalle 10, excluyéndose finalmente 3. La mayoría concluye con resultados estadísticamente significativos que la obesidad en pacientes con APs e inhibidores del TNF-α (iTNF-α) se asocia a una probabilidad menor de alcanzar y mantener la mínima actividad inflamatoria, con mayor tasa de interrupción del tratamiento y menor tasa de respuesta cutánea. En relación con los FAME sintéticos convencionales, se observó en obesos una tendencia a un aumento moderado de las transaminasas con metotrexato (MTX).
ConclusionesLa obesidad es un factor predictivo negativo de la respuesta clínica en pacientes con APs e iTNF-α. Exceptuando la hepatotoxicidad por el MTX, no se encontraron otros efectos adversos ni por otros fármacos en relación con la obesidad.
Obesity and its complications are common in patients with psoriatic disease.1–5 A number of epidemiological studies have identified a higher risk of developing certain metabolic alterations in patients with psoriasis and psoriatic arthritis (PsA), which also includes obesity. This is also because obesity and psoriasis seem to be linked by a common pathophysiological mechanism, which is explained by a chronic low-grade inflammation,2 with an increase in local and systemic inflammatory markers. In obesity, adipocytes show an imbalance resulting in an excessive secretion of the cytokines most detrimental from the cardiovascular point of view, such as interleukin (IL)-6, IL-18, tumor necrosis factor-alpha (TNFα) and leptin, as well as, a decreased release of protective cytokines, like adiponectin.2,6
It is known that obesity is associated with a higher incidence and severity of psoriasis, and that its presence affects the therapeutic response.7–9 This relationship is less widely studied in PsA. On the other hand, treatment with TNFα inhibitors (TNFαi) has been associated with an increase in weight both in psoriasis and in PsA,10 a fact that should be taken into account in the follow-up of these patients. It is for this reason that we propose studying whether obesity is associated with a higher incidence of adverse effects than treatments using an anchor drug in PsA, including the lack of response as an adverse effect.
MethodsThe study involved a systematic review of the literature, in a search for observational studies, using the guidelines of the MOOSE consensus.11
Search StrategyWe performed a search using Medline (via PubMed) and EMBASE databases (until 28 May 2015). The major PICO terms used for this analysis were “obesity” and “psoriatic arthritis”. Table 1 shows the terms utilized for the search. We introduced no limits concerning language, dates or ages, and only considered studies in humans.
Search strategies.
| Medline | EMBASE |
|---|---|
| “Obesity” [Mesh] Obesity [tw] Obesity [ti] Obesity [ab] “Overweight” [Mesh] “Overweight” [tw] “Overweight” [ti] “Overweight” [ab] “Body Mass Index” [Mesh] “Body Mass Index” [tw] “Body Mass Index” [ti] “Body Mass Index” [ab] “Body Weight/adverse effects” [Mesh] “Body Weight/drug effects” [Mesh] “Abdominal Obesities” OR “Abdominal Obesity” OR “Obesities, Abdominal” OR “Central Obesity” OR “Central Obesities” OR “Obesities, Central” OR “Obesity, Central” OR “Obesity, Visceral” OR “Obesities, Visceral” OR “Visceral Obesities” OR “Visceral Obesity” OR “Morbid Obesities” OR “Obesities, Morbid” OR “Obesity, Severe” OR “Obesities, Severe” OR “Severe Obesities” OR “Severe Obesity” OR “Morbid Obesity” (“Arthritis, Psoriatic” [Mesh] OR “psoriatic arthritis” OR (psoriasis AND arthritic) OR (arthritic AND psoriasis) OR “Psoriasis Arthropathica” OR (arthritis AND psoriasis)) | “Obesity”/exp “Overweight”/exp ––––––––––––– Weight gain/exp ––––––––– Adipose tissue hyperplasia Adipositas Adiposity Alimentary obesity Body weight, excess Fat overload syndrome Nutritional obesity Obesitas Overweight IMC (body mass index) Body ban mass Body mass index Quetelet index Weight increase ––––––– “Psoriasis” AND “arthritic” “Psoriatic” AND “arthritis” “Psoriasis” AND “arthritis” “Psoriatic arthritis”/exp OR “Psoriatic arthritis” |
The following selection criteria were established: (1) all the patients should have PsA or there should be an analysis differentiating patients with PsA; (2) the effect of the obesity factor or body mass index (BMI) should have been studied as a primary or secondary outcome measure; (3) the study had to be a clinical trial, a prospective or retrospective longitudinal or a case-control design, but series of case reports were not accepted; and (4) the outcome (primary measure) could be toxicity, whether specific or in general: hepatotoxicity (increase in enzymes, fibrosis, cirrhosis, hepatocarcinoma), insulin resistance, dyslipidemia, hypertension, hyperuricemia, a shorter survival than that attributed to the treatment or better therapeutic efficacy.
Two reviewers screened the articles according to title and abstract, and maintained for in-depth review any that offered doubts as to whether or not they complied with the selection criteria. We include the articles selected using this system, and record those that were not. The reason for exclusion is offered in the detailed review.
Variables and Data CollectionFrom the articles reviewed in detail, we gathered all the data from the sample description and the study objective, the design and duration of patient follow-up, the drugs employed, the definition of obesity, the variables utilized and their units of measure, with and without adjustment, as well as the variables included in the adjustment.
The quality was measured using an ad hoc risk of bias tool, that included: (1) design (low bias in prospective longitudinal and high bias in retrospective or case-control studies); (2) drug exposure time (high bias if the time is short, less than 3 months); (3) clarity in the definition of both the factor (obesity) and outcome (toxicity); and (4) effect measure (high bias if it is not adjusted).
AnalysisThe qualitative analysis of the information collected, depending on the study type and population studied, was done in terms of quality and specific results. We did a qualitative analysis of the heterogeneity.
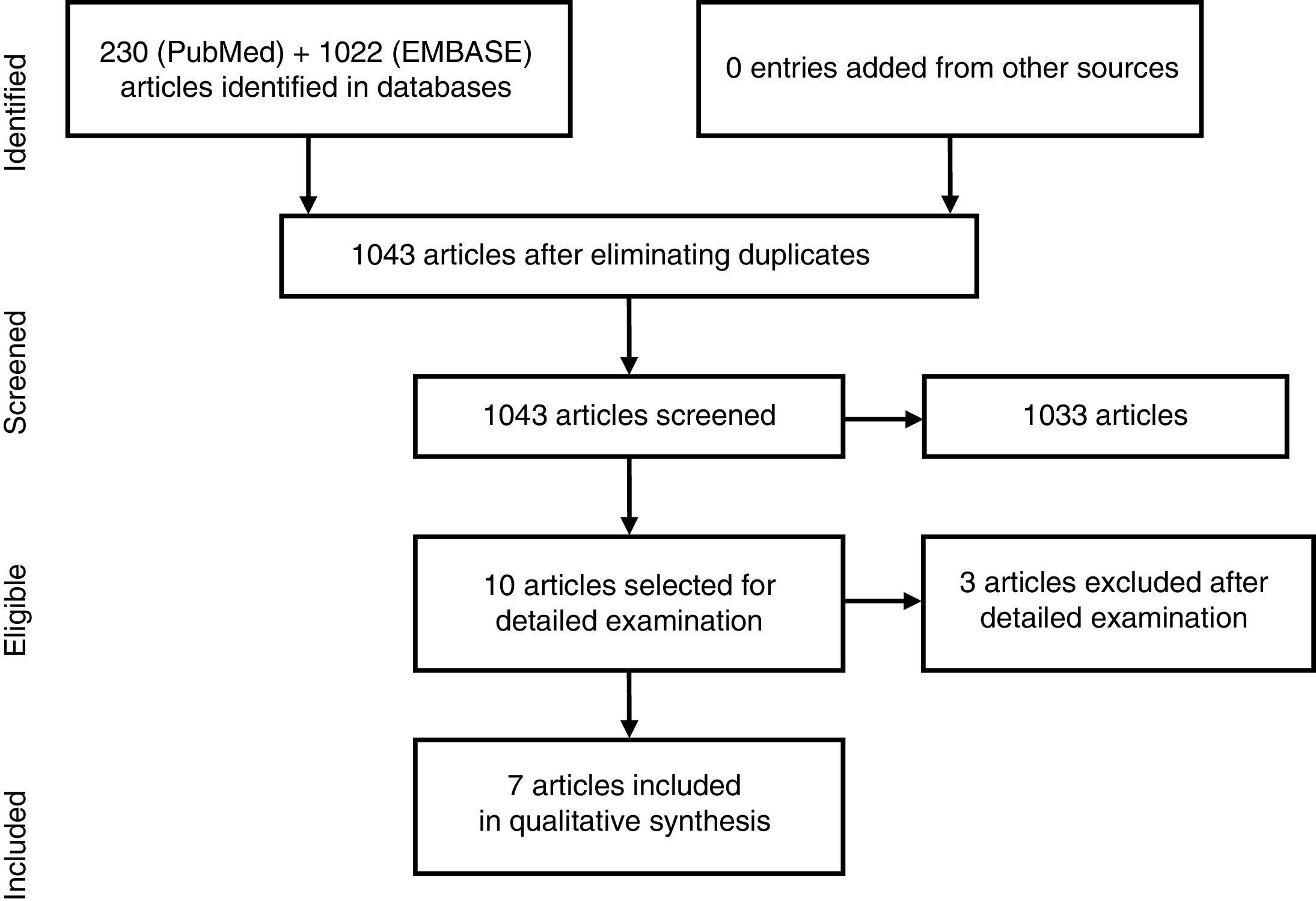
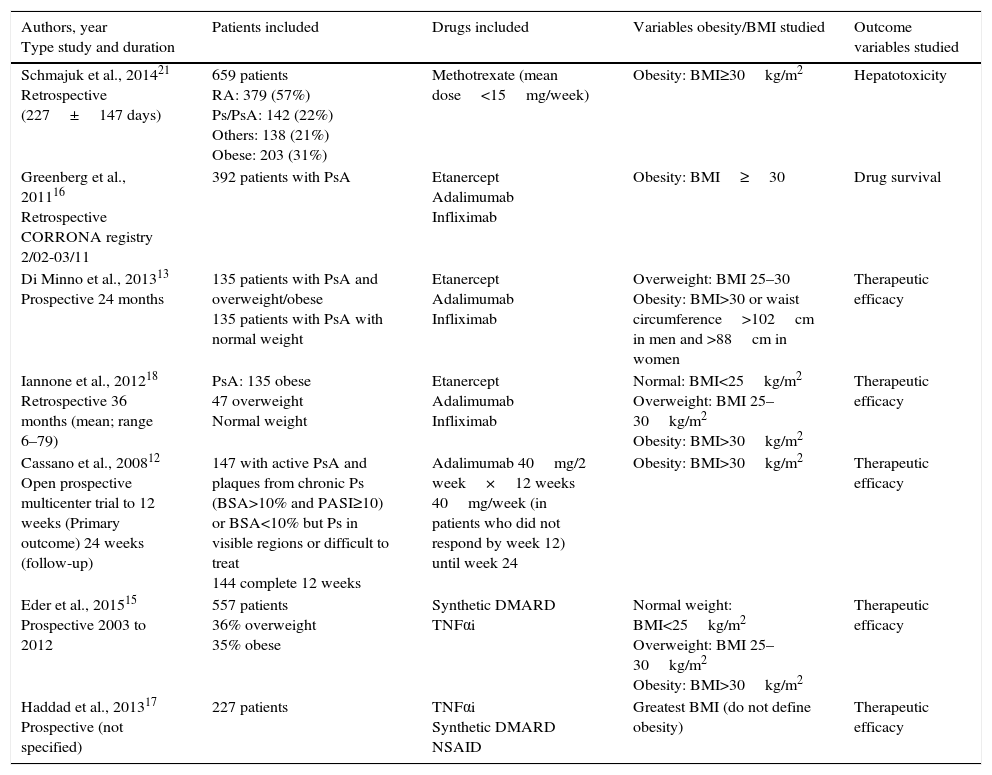
ResultsThe search strategy encountered 1043 titles, most of which were excluded on the basis of their title or abstract (Fig. 1). We reviewed 10 studies in detail,12–21 and excluded 3: that of Santiago García et al., as it was a cross-sectional study20; that of Koehm et al., because it did not include obesity among the predictors of adverse effects and efficacy failures19; and that of di Minno et al. of 2014, as it was really a clinical trial to see the effect of weight loss on activity, which is a different research question.14 Of the 7 included, 1 was a multicenter trial and the remainder were longitudinal observational studies (3 prospective and 3 retrospective) (Table 2).
Table Showing Evidence for Studies Included.
| Authors, year Type study and duration | Patients included | Drugs included | Variables obesity/BMI studied | Outcome variables studied |
|---|---|---|---|---|
| Schmajuk et al., 201421 Retrospective (227±147 days) | 659 patients RA: 379 (57%) Ps/PsA: 142 (22%) Others: 138 (21%) Obese: 203 (31%) | Methotrexate (mean dose<15mg/week) | Obesity: BMI≥30kg/m2 | Hepatotoxicity |
| Greenberg et al., 201116 Retrospective CORRONA registry 2/02-03/11 | 392 patients with PsA | Etanercept Adalimumab Infliximab | Obesity: BMI≥30 | Drug survival |
| Di Minno et al., 201313 Prospective 24 months | 135 patients with PsA and overweight/obese 135 patients with PsA with normal weight | Etanercept Adalimumab Infliximab | Overweight: BMI 25–30 Obesity: BMI>30 or waist circumference>102cm in men and >88cm in women | Therapeutic efficacy |
| Iannone et al., 201218 Retrospective 36 months (mean; range 6–79) | PsA: 135 obese 47 overweight Normal weight | Etanercept Adalimumab Infliximab | Normal: BMI<25kg/m2 Overweight: BMI 25–30kg/m2 Obesity: BMI>30kg/m2 | Therapeutic efficacy |
| Cassano et al., 200812 Open prospective multicenter trial to 12 weeks (Primary outcome) 24 weeks (follow-up) | 147 with active PsA and plaques from chronic Ps (BSA>10% and PASI≥10) or BSA<10% but Ps in visible regions or difficult to treat 144 complete 12 weeks | Adalimumab 40mg/2 week×12 weeks 40mg/week (in patients who did not respond by week 12) until week 24 | Obesity: BMI>30kg/m2 | Therapeutic efficacy |
| Eder et al., 201515 Prospective 2003 to 2012 | 557 patients 36% overweight 35% obese | Synthetic DMARD TNFαi | Normal weight: BMI<25kg/m2 Overweight: BMI 25–30kg/m2 Obesity: BMI>30kg/m2 | Therapeutic efficacy |
| Haddad et al., 201317 Prospective (not specified) | 227 patients | TNFαi Synthetic DMARD NSAID | Greatest BMI (do not define obesity) | Therapeutic efficacy |
BMI, body mass index; BSA; body surface area; DMARD, disease-modifying antirheumatic drug; NSAID, nonsteroid anti-inflammatory drug; PASI: Psoriasis Area Severity Index; Ps, psoriasis; PsA, psoriatic arthritis; RA, rheumatoid arthritis; TNF-αi, tumor necrosis factor α inhibitor.
The study of Greenberg et al.16 analyzes whether obesity contributes to treatment survival and that of Schmajuk et al.21 the effect of obesity on transaminases. The remainder of the studies include analyzing the effect on patient response. According to our risk of bias scale, the designs are appropriate but, except in that of Schmajuk et al., the outcome variables are not exactly toxicity.
The population studied included patients with active PsA and different degrees of BMI and mostly treated with TNFαi. The majority of the studies define obesity as a BMI>30kg/m2, although, in Haddad et al.,17 it is not defined. With regard to outcome measures, the authors mostly study the percentage of patients with minimal disease activity (MDA), but there are other response measures in PsA.
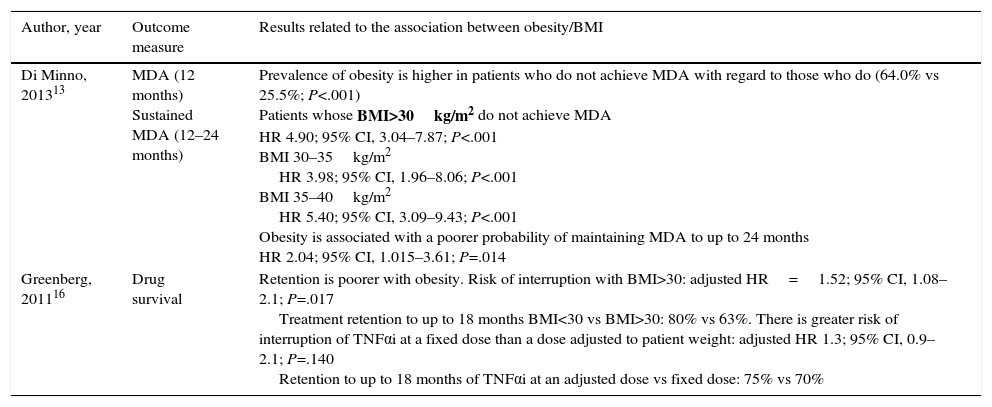
Four studies reveal the impact of obesity on the clinical response of patients with PsA being treated with TNFαi. Three of them conclude with statistically significant results indicating that overweight and obesity are associated with a lower rate of achieving MDA and maintaining it over time. In their clinical trial, Cassano et al. evaluate Psoriasis Area and Severity Index (PASI-50), with results that show significantly that the rate of response of psoriasis to treatment with adalimumab is lower in patients with a BMI>30kg/m2.12 The study of Iannone et al.18 is the only article in which BMI does not appear to influence the rate of remission of the disease in patients with PsA and TNFαi therapy (for a summary of the results, see Table 3).
Results of the Effect of Obesity on Patient Response.
| Author, year | Outcome measure | Results related to the association between obesity/BMI |
|---|---|---|
| Di Minno, 201313 | MDA (12 months) Sustained MDA (12–24 months) | Prevalence of obesity is higher in patients who do not achieve MDA with regard to those who do (64.0% vs 25.5%; P<.001) Patients whose BMI>30kg/m2 do not achieve MDA HR 4.90; 95% CI, 3.04–7.87; P<.001 BMI 30–35kg/m2 HR 3.98; 95% CI, 1.96–8.06; P<.001 BMI 35–40kg/m2 HR 5.40; 95% CI, 3.09–9.43; P<.001 Obesity is associated with a poorer probability of maintaining MDA to up to 24 months HR 2.04; 95% CI, 1.015–3.61; P=.014 |
| Greenberg, 201116 | Drug survival | Retention is poorer with obesity. Risk of interruption with BMI>30: adjusted HR=1.52; 95% CI, 1.08–2.1; P=.017 Treatment retention to up to 18 months BMI<30 vs BMI>30: 80% vs 63%. There is greater risk of interruption of TNFαi at a fixed dose than a dose adjusted to patient weight: adjusted HR 1.3; 95% CI, 0.9–2.1; P=.140 Retention to up to 18 months of TNFαi at an adjusted dose vs fixed dose: 75% vs 70% |
| Iannone, 201218 | Normal weight | Overweight | Obese | P | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |||
| HAQ | 0.79 (0.9) | 0.5 (1.2) | 0.47 (0.8) | 0.0 (0.7) | 0.81 (0.8) | 0.75 (1.5) | .06 | |
| DAS28 | 3.1 (1.6) | 2.7 (1.5) | 2.9 (1.6) | 2.7 (2.7) | 3.2 (1.5) | 3.3 (2.4) | .42 | |
| SDAI | 14.2 (13) | 14.7 (11) | 11.6 (12) | 8.0 (17) | 13.0 (12) | 14.0 (6) | .44 | |
| DAS28<2.6 | 44% | 46% | 37% | .31 | ||||
| SDAI<3.3 | 21% | 38% | 21% | .07 | ||||
| EULAR response | 61.5% | 63.8% | 62.8% | .05 | ||||
| Cassano, 200812 | PASI 50 | BMI<30 | BMI>30 | P |
|---|---|---|---|---|
| 79% | 58% | .02 | ||
| Eder, 201515 | MDA sustained for at least 1 year | BMI 25–30 | BMI>30 | |
| OR | 0.64 | 0.52 | ||
| P | .001 | .0001 | ||
| Haddad, 201317 | MDA | 146 patients achieved MDA after a mean duration of 1.3 years. Patients who did not achieve MDA had a baseline BMI>31.6 vs 28.5; P=.02 | ||
BMI, body mass index; CI, confidence interval; DAS28, Disease Activity Score; EULAR, European League Against Rheumatism; HAQ, Health Assessment Questionnaire; HR, hazard ratio; IQR, interquartile range; MDA minimal disease activity; OR, odds ratio; PASI, Psoriasis Area Severity Index; SD, standard deviation; SDAI, Simplified Disease Activity Index; TNFαi; tumor necrosis factor α inhibitor.
The study of di Minno et al.13 includes 135 patients with PsA who are overweight and/or obese and 135 with PsA and a normal body weight. The prevalence of obesity is higher in patients who do not achieve MDA after 12 months (64.0% vs 25.5%; P<.001), and is associated with a lower rate of maintaining it after 24 months (hazard ratio [HR] 2.04, 95% confidence interval [CI], 1.015–3.61; P=.014).
The study of Iannone et al.18 did not have the same results and, as limitations, it is retrospective, the patient cohort is smaller and the characteristics of the patients are different (they have no axial involvement, a very low PASI and very few had a second degree of obesity).13
The study by Greenberg et al.,16 with data from the CORRONA registry, evaluates the influence of weight and BMI in the persistence of TNFαi therapy in patients with PsA and observes that the risk of interruption is significantly greater in patients with a BMI≥30. The adjusted risk of interruption of the treatment in persons with a BMI≥30 is HR=1.52 (95% CI of 1.08–2.1); P=.017, with persistence at 18 months of 80% vs 63% in obese individuals. Patients with a fixed dose of TNFαi showed a higher risk of interruption (the cause is not specified) than those with a dose established in relation to their weight, with an adjusted HR of 1.3 (95% CI of 0.9–2.0) and the persistence of 75% vs 70% at 18 months. Additionally, they evaluate other variables that determine the risk of interruption of the treatment, including pain during their baseline visit, gender, previous disability and cardiovascular comorbidity, with significant differences regarding gender (woman vs man; P=.004), baseline pain (≥4 vs <4; P=.026) and cardiovascular comorbidity (P=.007).
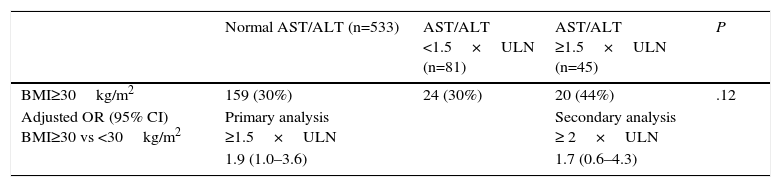
Finally, in the study of Schmajuk et al.,21 which includes 659 patients, 142 (22%) of whom, had psoriasis/PsA and 31% had a BMI≥30.0kg/m2, there was a trend toward a moderate increase in transaminases in obese patients receiving methotrexate (Table 4).
Results for Hepatotoxicity in the Study by Schmajuk et al., 2014.21
| Normal AST/ALT (n=533) | AST/ALT <1.5×ULN (n=81) | AST/ALT ≥1.5×ULN (n=45) | P | |
|---|---|---|---|---|
| BMI≥30kg/m2 | 159 (30%) | 24 (30%) | 20 (44%) | .12 |
| Adjusted OR (95% CI) BMI≥30 vs <30kg/m2 | Primary analysis ≥1.5×ULN | Secondary analysis ≥ 2×ULN | ||
| 1.9 (1.0–3.6) | 1.7 (0.6–4.3) |
AST upper limit of normal=35mg/mL. ALT upper limit of normal=60mg/mL.
Abnormality: mild<ULN×1.5; moderate≥ULN×1.5; severe>ULN×10.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; OR, odds ratio; ULN, upper limit of normal.
Taken from Schmajuk et al.21
This review concludes that obesity is associated with a higher risk of not achieving and maintaining MDA in PsA and is, in short, a negative predictive factor of the clinical response to TNFαi.12 There are tests with a moderate risk of bias in which obesity is associated with a shorter survival of drugs with TNFαi. This is probably due more to a lack of efficacy for the reasons mentioned above rather than for toxicity. A shorter survival was also observed with TNFαi drugs administered with a fixed dose, as was shown by the study of Greenberg et al.16
However, the study of di Minno et al.,13 in accordance with the results (MDA achieved in 15.6% of the patients with infliximab, 21.3% with etanercept and 18.6% with adalimumab), we must decide whether obesity should be considered a determining factor in the indication for treatment with TNFαi with doses related to the weight of patients with PsA.
In the clinical trial published by di Minno et al.,14 ultimately, excluded from this review, it is observed that a loss of weight (≥ 5% with respect to the baseline weight) is associated with a greater probability of achieving MDA at 6 months (odds ratio [OR]=4.20; 95% CI, 1.82–9.66; P<.001). Moreover, this probability of achieving MDA increases proportionally to the loss of weight; weight losses of <5%, 5%–10% and >10% are associated with a MDA of 23.1%, 44.8% and 59.5%, respectively.
Likewise, in obese patients with PsA receiving methotrexate, there has been evidence of a moderate increase in transaminases. Unfortunately, we have found no article that approaches adverse effects other than hepatotoxicity or with other drugs. The case is that the question remains only partially answered. On the other hand, the development of hepatic steatosis does not appear to be influenced by biologic therapy in patients with PsA and is related more to risk factors, like metabolic syndrome and the activity of the disease itself.22
In short, obesity is a negative predictive factor of the clinical response in patients with PsA to drugs with TNFαi and, probably, of a somewhat more serious hepatotoxicity because of methotrexate. We found, with an evidence level of 3, that obesity is associated with a less marked therapeutic response to drugs with TNFαi and, probably to other treatments for PsA. Thus, obesity should be considered an indication for adjusting the dose of those drugs to the weight of the patient. Likewise, and although our studies must be confirmed, obesity is still associated with a moderate increase in transaminases in patients being treated for PsA with methotrexate. Therefore, the patient should intend to lose weight or watch his or her liver enzymes, or both.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingMerck Sharp & Dohme España financed the performance of the systematic review as part of a global document offering recommendations concerning the management of comorbidities in PsA.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Galíndez E, Carmona L. ¿Se asocia la obesidad en la artritis psoriásica a una menor respuesta terapéutica y más efectos adversos con el tratamiento de fondo? Reumatol Clin. 2016;12:307–312.