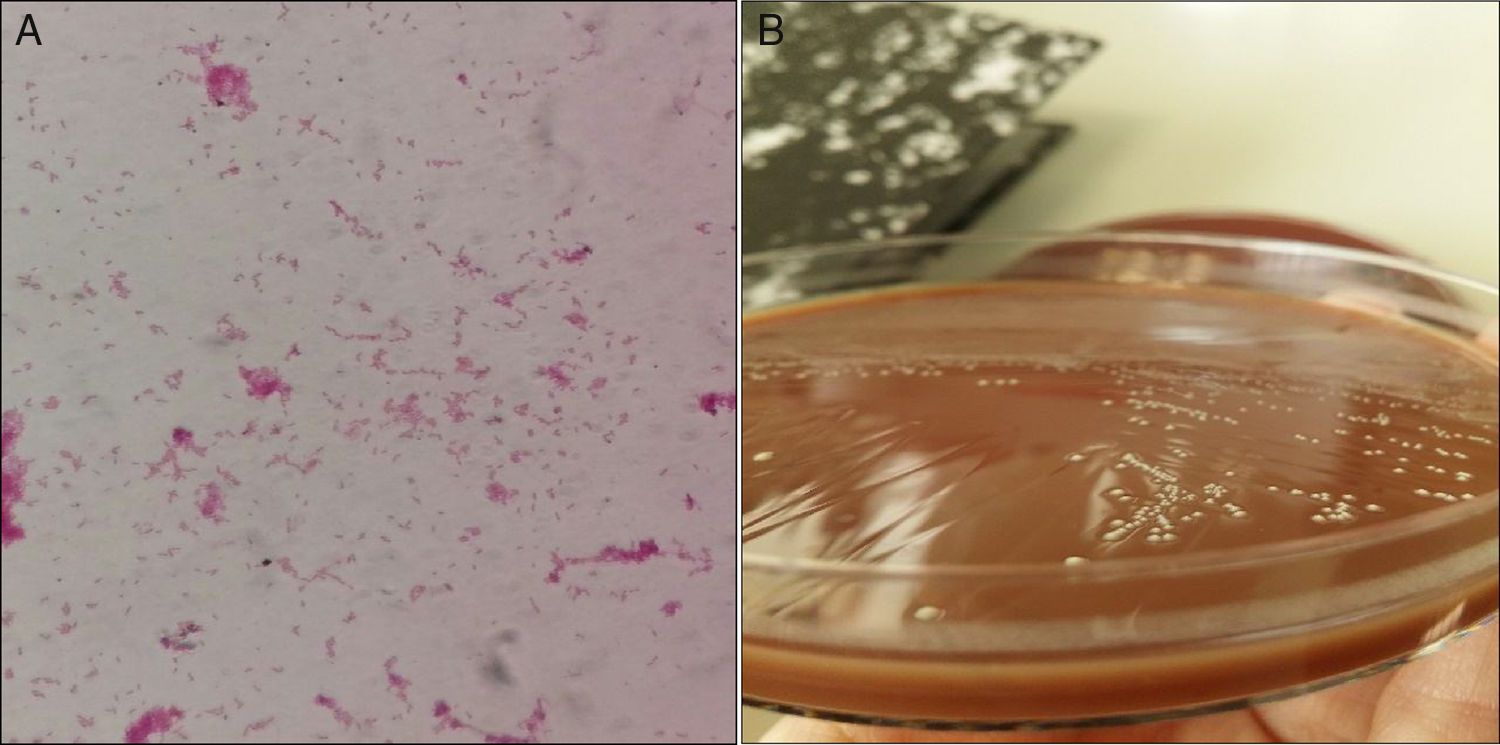
Streptococcus mutans (Sm) is a microorganism of oral microbiota, which causes dental decay, bacteriaemia and endocarditis. Only a single case report of arthritis from this microorganism1 has been published.
We present a case of joint prosthesis infection by Sm. A 73 year-old male presented with distal oedemas and cellulite in the lower left limb (LLL). The patient had a history of morbid obesity, high blood pressure, dyslipidemia, type 2 diabetes mellitus and chronic renal disorder. Total right and left hip replacements had been performed (1998–1999); left knee replacement (2014) due to medial tibiofemoral compartment gonarthrosis in varus knee. He had non complicated cellulite in LLL 2 years previously. There was no history of oral treatment. Findings from Venus Doppler Ultrasound were: oedema of the subcutaneous cell tissue in LLL, with joint effusion in the anterior compartment of the knee. Possible infection of the total prosthesis was diagnosed and cellulite of the left knee; an extraction of the cement spacer with extraction of the tibial and femoral component was performed. The patella polyethylene was removed and the fragmented external side was extracted. The cemented knee prosthesis was positioned, with semi-constrained, fixed plates, SAMO trecking; with no patella prosthesis. There were no clinical signs or laboratory analysis compatible with sepsis or bacteriaemia. No clinical or radiological signs were compatible with osteomyelitis. One hundred cc of seropurulent liquid was obtained by evacuative arthrocentesis. Inoculated synovial fluid was sent in blood culture bottles to the microbiology laboratory. Antibiotic therapy with intravenous vancomycin and rifampin was initiated. After 30h of incubation in Bactec 9240 (BD) Sm was identified directly from aerobic bottle, using mass spectrometry (MALDI-TOF®, Bruker). In Gram (Fig. 1A) staining, pleomorphic variable gran bacilli were observed. The appearance of the colonies is represented in Fig. 1B. The direct bottle phenotype identification (panel WalkAway®, Beckman) the following 48h was compatible with the identification of MALDI-TOF®. Antibiotic sensitivity was undertaken in addition to micro dilution (panel WalkAway®), using concentration gradient (E-test®, Bio-Merieux), following the directives of EUCAST version 6.0 2016-01-01. The microorganism was sensitive to penicillin, cefotaxime, clindamycin and vancomycin. Minimum inhibitory concentration (MIC), levofloxacin≤1, linezolid≤1 and daptomycin≤.5.
Twelve days after hospital admittance surgery was performed, with synovial fluid being taken again and the re-identification of Sm.
The patient's evolution was favourable with obvious clinical improvement compared with his pre-surgical status and after 18 days the patient was discharged from hospital with an outpatient antibiotic treatment (levofloxacin: 500mg/12h oral administration for 42 days).
Sm is a pleomorphic streptococcus (bacillary in an acidic medium and coccus in a neutral or alkaline medium). Described by Clarke in 1924 (tooth decay) and later its capacity to produce endocarditis was observed. The Sm group includes isolated species in humans (mutans, sobrinus, criceti, ratti and downei) and other isolated species in animals. The mutans species is the producer of caries and the mother–child transmission is probably through oral secretions, with this species being the one most frequently associated with bacteriaemia. It may be confused with other streptococci and enterococci. Its isolation and identification may be difficult, as was corroborated by Emmerson and Eykyn, and also by Schelenz and Cois, reporting cases of endocarditis in which the agent had been erroneously determined as diphtheroid.1–3 It is resistant to phagocytosis by polymorphonuclear leukocytes4 and may participate in the pathogenesis of atherosclerosis.5 Many reports have been published associating Sm with the production of biofilm in dental plaque and in materials used in odontology,6 but there are few or no studies regarding Sm adherence to material used in prostheses or osteoarticular tissues. It has been suggested that the bacteria wall could contribute to the inducement of arthritis in animal models.7 The bacterial protein I/II (pathogen-associated molecular pattern [PAMP]) could contribute to adverse evolution in osteoarthritis.8 It has been described that non C serotypes are prone to extra-oral translocation.9 It has been associated with conditions such as Behçet's disease, Sjögren syndrome and rheumatoid arthritis.4,8
Few infections of the joints have been reported. Before molecular identification techniques (sequencing) and proteomic techniques were available (MALDI-TOF®), identification posed a diagnostic challenge due to Sm's ability to present as a “gram positive bacillus” to Gram stain and its possible confusion with other microorganisms.
Please cite this article as: Parra-Grande M, Hernández-Ros P, Prats-Sánchez I, López-García P. Infección de prótesis articular por Streptococcus mutans. Reumatol Clin. 2019;15:e78–e79.