A 50-year-old woman with systemic lupus erythematosus treated for 13 years with hydroxychloroquine developed nephropathy and high blood pressure 5 years ago as well as moderate loss of vision in her right eye. Fundoscopy showed alterations of macular pigmentation only in the right eye. Visual fields 10-2 were normal in both eyes. Optical coherence tomography showed hyperreflective foveal thickening with a hyporreflective cavity underlying in the right macula, and was normal in left macula. Fluorescein angiography showed no bulls-eye pattern, but did show microaneurysms in vascular arcades. Multifocal central electroretinogram was diminished in right eye and the electroretinogram pattern was diminished in both eyes. We concluded that the alterations of the right eye were suggestive of ischemic maculopathy, not chloroquine toxicity.
Una mujer de 50 años con lupus eritematoso sistémico, tratada 13 años con hidroxicloroquina y desde hace 5 años con nefropatía e hipertensión arterial, refirió en la revisión periódica pérdida moderada de visión en el ojo derecho. La fundoscopia mostró solo alteraciones de la pigmentación macular en el ojo derecho. El campo visual 10-2 fue normal en ambos ojos. La tomografía de coherencia óptica mostró en la mácula derecha un engrosamiento foveal hiperreflectivo, con cavidad hiporreflectiva subyacente, y fue normal en la mácula izquierda. La angiografía fluoresceínica no mostró patrón en ojo de buey, sino microaneurismas en arcadas vasculares. El electrorretinograma multifocal central estaba disminuido en el ojo derecho y el electrorretinograma patrón moderadamente disminuido en ambos ojos. En conclusión, las alteraciones del ojo derecho fueron indicativas de maculopatía isquémica, pero no de toxicidad cloroquínica.
Synthetic antimalarials in the treatment of systemic lupus erythematosus (SLE) have been a major therapeutic advance and serve as immunomodulators and inhibitors of thrombosis, with few side effects, prolonging the quality of life of patients. Their withdrawal leaves the patient with SLE at the mercy of the side effects of corticosteroids and immunosuppressants, and increases the risk of flares.1
Hydroxychloroquine can cause severe retinal toxicity, requiring discontinuation of therapy, although there are few cases of cloroquine retinopathy, always related to very high cumulative drug dose.2
Synthetic antimalarial toxicity is detected by ophthalmic screening protocols focused on development of macular pathology.3 One must not forget that the patient with SLE is predisposed to retinal ischemia that may be confused with macular damage due to cloroquine. We present a case in which the ocular findings represented a challenge in the therapeutic decision.
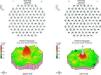
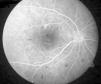
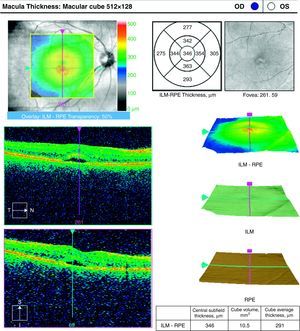
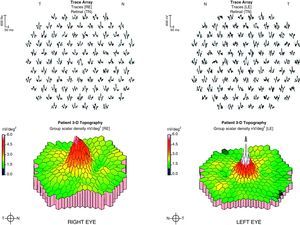
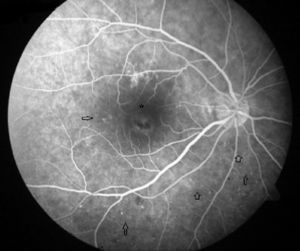
Case ReportA 50-year-old woman with SLE was treated with a umulative dose of 365g of hydroxychloroquine for 13 years, with mild lupus nephritis and hypertension controlled 5 years prior; during her screening for ocular toxicity she presented moderate loss of vision, presenting a corrected visual acuity of 0.7 in the right eye and 1 in the left eye. In the right fundus we observed irregular areas of hyperpigmentation and depigmentation in the macula, without apparent serous uprising, and some arcades microhemorrhages. The left fundus appeared normal. 10-2 the visual field was normal in both eyes. Optical coherence tomography (OCT) of the right eye showed hyperreflective foveal thickening and hyporreflective cavities in deep layers (Fig. 1). The OCT of the left eye was normal. Fluorescein angiography (FA) showed no bull's-eye pattern, but microaneurysms in both eyes (not detected on fundoscopy), sectoral distribution and hyper-and hypofluorescence late macular leakage (Fig. 2). In the multifocal electroretinogram (mfERG) there were low amplitude responses in both peripheral retinas, low power density in the foveal region of the right eye and normal foveolar activity in the left eye (Fig. 3). The alteration of the N95 wave of the electroretinogram pattern reflected a dysfunction of the ganglion cells of both retinas, probably causing the disease.
The neurophysiological findings, the OCT and FA suggested a vascular origin rather than a toxic effect, so it toxicity was ruled out and hydroxychloroquine retinopathy was diagnosed as lupus, recommending the maintenance of the antimalarial but with close monitoring of the patient to prevent maculopathy as a risk factor. In subsequent visits the patient maintained her visual acuity and had improved lupus symptoms with hydroxychloroquine maintenance treatment, which confirmed the suspected end diagnosis.
DiscussionIn the early alterations of the visual field as a sign of cloroquine maculopathy 10-2, central mfERG, OCT and autofluorescence have recently been added as diagnostic tests, with their use recommended for routine screening.3 Central mfERG shows very early alterations,4 but its availability is scarce.
The field of view of our patient was normal in both eyes, which ruled cloroquine maculopathy. The mfERG showed macular dysfunction only in the right eye and asymmetry is rare in drug toxicity. The results of the AGF and the rest of neurophysiological studies suggested an episode of retinal ischemia. The OCT image was reminiscent of a rare form of central serous chorioretinopathy, lupus5 choroidopathy, while microaneurysms in cuneiform distributions were more typical of hypertensive episodes.
Faced with a maculopathy in a patient with SLE treated with hydroxychloroquine, we must not forget that they are patients exposed to thrombotic events that can generate retinal confusion, so that additional tests may be necessary to make an adequate differential diagnosis, because without maculopathy there is no need for drug withdrawal, even to assess an increase in dosage.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of InterestThe authors have no disclosures to make.
Please cite this article as: Rodríguez-Hurtado FJ, et al. Maculopatía en paciente con lupus eritematoso sistémico tratado con hidroxicloroquina. Reumatol Clin. 2012. doi:10.1016/j.reuma.2011.12.012