The AR Excellence project evaluates clinical monitoring in patients with rheumatoid arthritis (RA) in Spain. The aim of the study was to analyse the use of methotrexate (MTX) in the AR Excellence cohort and to compare it with current recommendations.
Patients and methodsWe collected data from RA patients who initiated treatment with MTX. They included demographics, dose and routes of administration, switching among them, highest dose in each route, combinations with other disease-modifying antirheumatic drugs (DMARDs), time to combination with another DMARD (either conventional or biological) and adverse events.
ResultsSix hundred twenty-five patients with RA (mean age 55 years; 70.6% women) were included, with an average disease duration of 21 months. Ninety percent of the patients initiated treatment with MTX. Therapy was begun with a mean dose of 11mg per week; this initial dose was increased in 58% of the individuals. The average time to reach the full dose of MTX (20mg a week) was 6.67 months. Time to combination of MTX with another DMARD, either synthetic or biological, was 3 months. In all, 67.4% of the patients received oral MTX and the route was subcutaneous in 18.6%. In 12% of the cases, there was a change in the route of administration after a period of 6 months. In 544 patients, folate supplements were added to MTX; MTX-related adverse events were detected in 17.3% of the patients.
ConclusionMTX is currently the pivotal treatment in RA. The subanalysis of the AR Excellence project demonstrates that MTX escalation to its full doses is not done with adequate speed. The subcutaneous route is used in a small proportion of patients.
El proyecto AR Excellence evalúa la atención clínica a los pacientes con artritis reumatoide (AR) en España. El objetivo del presente estudio es analizar la utilización de metotrexato (MTX) en AR Excellence y compararla con las recomendaciones vigentes.
Pacientes y métodosSe revisó a pacientes con AR que habían iniciado tratamiento con MTX, recogiendo datos demográficos, dosificación, vías de administración, combinaciones con otros fármacos antirreumáticos modificadores de enfermedad (FAME), tiempo hasta combinación con otro FAME (convencional o biológico) y efectos adversos.
ResultadosSe incluyó a 625 pacientes con AR (edad media de 55,1 años; 70,6% mujeres), con una duración media de la AR de 21,3 meses. El 90% inició tratamiento con MTX. La dosis media de inicio fue de 11mg semanales; en el 58% de los casos se incrementó la dosis. El tiempo medio hasta alcanzar la dosis plena de MTX (20mg semanales) fue de 6,67 meses. El tiempo hasta la combinación de MTX con otro FAME sintético o biológico fue de 3 meses. El 67,4% de los pacientes recibieron el MTX por vía oral y el 18,6%, subcutáneo. En el 12% de los casos se cambió la vía de administración, transcurrida una media de tiempo de 6 meses. En 544 pacientes se asociaron suplementos de folato. El 17,3% de los sujetos presentaron acontecimientos adversos por MTX.
ConclusiónEl MTX es el fármaco sobre el que pivota el tratamiento de la AR. El subanálisis del proyecto AR Excellence nos informa de que la escalada a sus dosis plenas no se realiza con la rapidez adecuada. La vía subcutánea se utiliza en pocos pacientes.
Despite many recommendations regarding the use of methotrexate (MTX) in rheumatoid arthritis (RA), there is still currently much variation in its clinical management. Aspects including dosing, dose escalation and de-escalation, administration route, concomitant use of folic acid and the impact of the drug when combined with biologic agents are highly differently reported in the different studies and there is also little information on them in Spain.1–6
The Spanish Society of Rheumatology (SER) has carried out the RA Excellence Project, the aim of which is to evaluate the quality of clinical care to patients with RA.7 The RA Excellence database contains a great deal of information on the use of MTX in real clinical practice. This has allowed us to analyse it and compare it with the current recommendations on the use of this drug.4,6,8–11 The aim of this sub-study was to assess the clinical use of MTX in patients with RA in Spain, using the information contained in the RA Excellence.
Patients and MethodsDesignThe RA Excellence project is a descriptive, retrospective and multicentre study carried out in the Rheumatology department of Spanish hospital centres. The aim of this project is to assess the care quality of patients with RA through a composite indicator constructed from a combination of consensual quality indicators by a group of experts using a Delphi 2-round technique and with bias from clinical relevance criteria and feasibility.12
Centre Selection and RecruitmentIndividuals were invited to participate from all rheumatology services of Spanish centres (public and private) recorded on the SER database, which collects information on over 90% of Spanish hospital centres. Recruitment was chronologically consecutive, according to the order of arrival of the request to participate in the assessment process. Participation of the centres was voluntary. The total of centre participants was 34, distributed throughout Spanish territory. Services where the rate of RA was under 5 cases per 100,000 individuals/per year were excluded. The assessment of care quality in rheumatology services was carried out between October 2014 and June 2015. Approval from the ethics committee and the clinical research department of the University Hospital Puerta de Hierro-Majadahonda, of the community of Madrid was obtained.
Collection of Information and Sample Size lInformation was collected for the assessment of quality markers from medical files of patients over 18 years of age diagnosed with RA according to the 2010 ACR/EULAR criteria13 between 1st January 2010 and 31st December 2013 in an external rheumatology consultation. The patients included had to continue in follow-up in the Rheumatology Service where they carried out the diagnosis until 31st December 2013. The medical files of patients with RA who had taken part in clinical trials during the previously mentioned period were excluded. Equally, those medical files where it was impossible to collect information for any reason were excluded. The sample size was estimated for meeting with the weighted composite indicator, assuming a binomial distribution. Nineteen medical files were selected by each Rheumatology Service included, to guarantee a minimum success rate of 75% with one failure (non compliance) and a sample error of .0268.
The sample was made randomly, using replacements to reach the sample size per centre. Each hospital provided the total number of medical files for the patients who met with the selection criteria and from numerical randomisation, the medical files to be reviewed were selected. Information was collected in a data collection logbook (DCL) by 2 qualified and trained external professionals. The DCL was designed and agreed upon by the main researcher and the scientific committee of the project. This information was later inserted into a database in SPSS format for result analysis. The DCL was piloted in 19 medical files of one centre.
VariablesVariables collected for this study were as follows: patients with RA who began treatment with MTX, initial dose of MTX, patients with an increase or reduction in MTX dose, time up until full dose of MTX (20mg/week), patients in whom adverse effects appeared associated with MTX, patients in treatment with folic or folinic acid combined with MTX, patients with a final MTX dose under 7.5mg/week, MTX administration route, change of MTX administration route throughout the course of treatment, time until the change in the administration route and time until the combination with another DMARDs (conventional or biologic).
Statistical AnalysisThe numerical variables with a normal distribution were expressed with means and standard deviation (SD) and the asymmetrical numerical variables with mean and interquartile range (p25–p75). Absolute and relative frequencies were calculated for the qualitative variables. Data management and statistical analysis management were focused on the SER Research Unit, in keeping with a prefixed analysis plan. All analysis was undertaken using the statistical analysis SPSS 21.0 software. A statistical significance level of under .05 was used.
ResultsThe study included 625 patients with RA from 34 rheumatology services in Spain, with the patient mean per centre being 18.1 (SD: 1.3). Mean age was 55.1 years (SD: 15.2); 441 patients were women (70.6%) and 184 (29.4%) were men. Mean duration of the disease was 21.3 months (SD: 13.4). Six hundred and fourteen individuals (98.2%) had a positive serum rheumatoid factor and 565 (90.4%) were carriers of antibodies against anti-citrullinated peptide antibodies (ACPA). 17.9% of patients were active smokers.
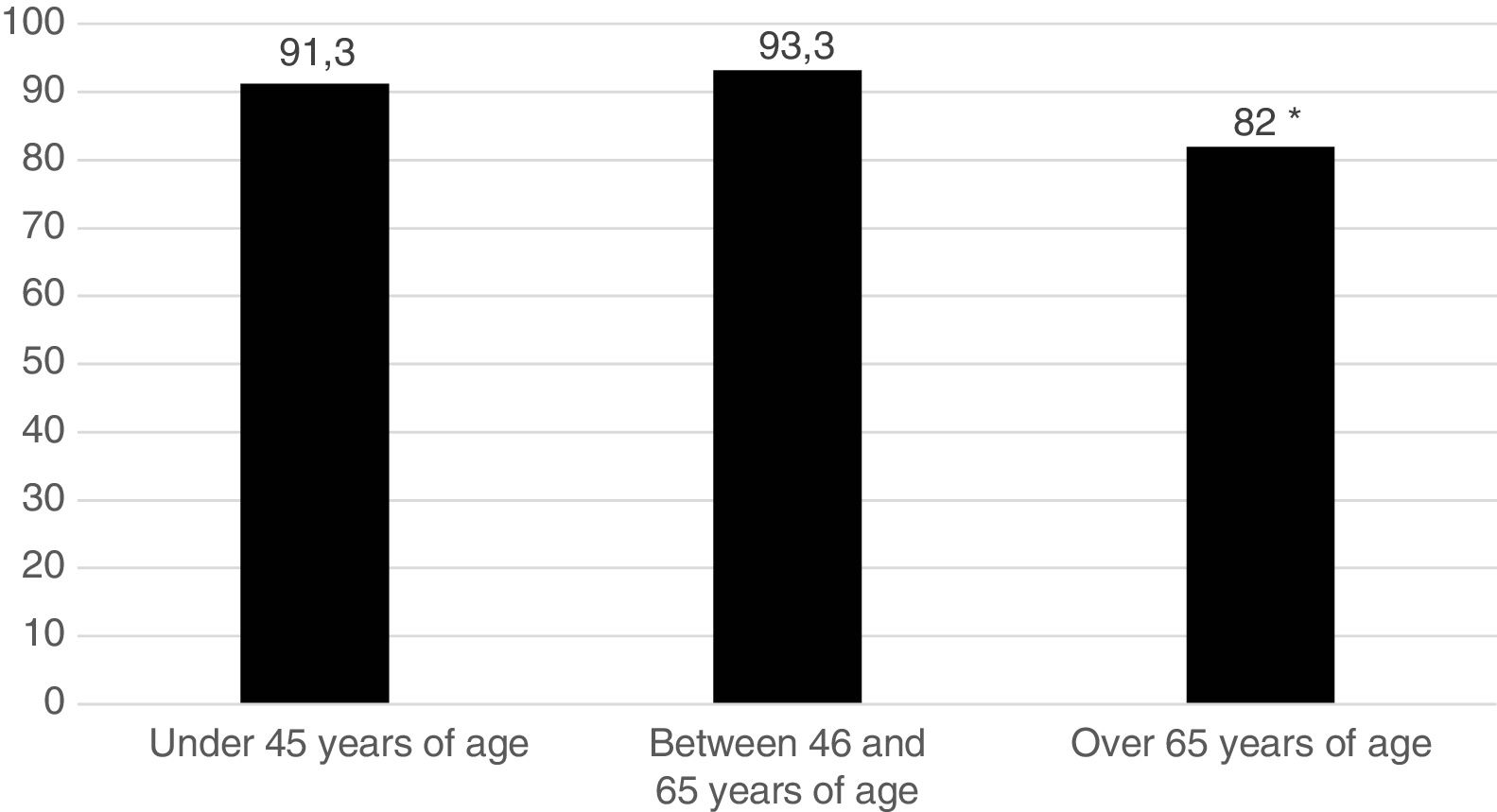
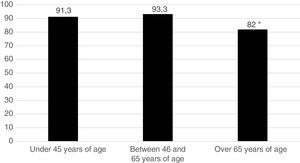
Five hundred and sixty one patients (89.8%) began treatment with MTX (90.7% of the women and 87.5% of the men). Fig. 1 represents the start of treatment with MTX according to the different patient age groups and we may observe that the group in which the indication of the drug is lower is the group over 65 years of age. No patient used sulfasalazine or anti malaria medicine in combined treatments. DMARDs were used in monotherapy: 134 patients (21.4%) received hydroxychloroquine and 29 patients (4.6%), sulfasalazine. Treatment strategy by T2T objectives was used. The mean pre-treatment value with MTX of the DAS28 was 4.66; 98.2% of patients were rheumatoid factor positive and 90.4% were ACPA positive.
The mean dose at the beginning of therapy with MTX was 11mg weekly (SD: 3.2); in 58.2% of patients this initial dose was increased and in 8% of patients it was reduced. Mean time for reaching full dose of MTX (20mg weekly) was 6.67 months (SD: 3.75, median=3.75 [1.83–9.16]) and only 15 patients (2.7%) presented at the end of the therapeutic period with a lower dose MTX to 7.5mg weekly. 5.3% of patients combined MTX with leflunomide and 3.4% with a biologic DMARD. The time up until the combination of MTX with another synthetic or biologic DMARD was 3 months (interval 0–11 months). 67.4% of patients received MTX orally and 18.6% subcutaneously; in the remaining 14% it was not possible to obtain the initial administration route of the drug. In 11.85% of cases there was a change of administration route, with a mean time of 5.7 months passing (interval: 2.7–9.6 months). When passing on to 15mg, 33.3% of patients received oral MTX, 38.9% subcutaneous MTX and we are not aware of the MTX administration route for 27.7% of patients. The information on results is contained Tables 1–3. Sufficient data was not obtained to be able to relate the dose changes or the administration route of MTX with levels of disease activity. It was not possible to analyse the changes in MTXC use on the basis of hospital type or region.
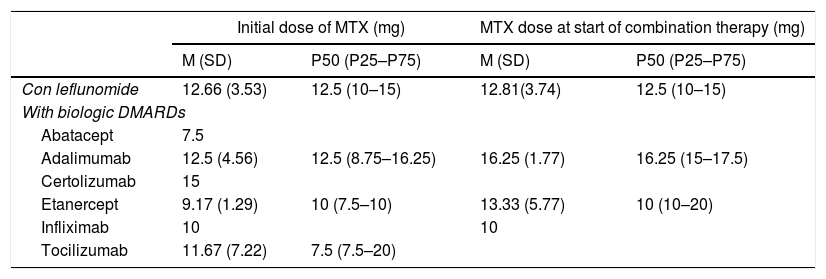
MTX Dosing in Combination With Leflunomide and With Different Biologic DMARDs: Initial Dose of a MTX in Monotherapy and Dose When Beginning Combined Therapy.
| Initial dose of MTX (mg) | MTX dose at start of combination therapy (mg) | |||
|---|---|---|---|---|
| M (SD) | P50 (P25–P75) | M (SD) | P50 (P25–P75) | |
| Con leflunomide | 12.66 (3.53) | 12.5 (10–15) | 12.81(3.74) | 12.5 (10–15) |
| With biologic DMARDs | ||||
| Abatacept | 7.5 | |||
| Adalimumab | 12.5 (4.56) | 12.5 (8.75–16.25) | 16.25 (1.77) | 16.25 (15–17.5) |
| Certolizumab | 15 | |||
| Etanercept | 9.17 (1.29) | 10 (7.5–10) | 13.33 (5.77) | 10 (10–20) |
| Infliximab | 10 | 10 | ||
| Tocilizumab | 11.67 (7.22) | 7.5 (7.5–20) | ||
SD: standard deviation; DMARDs: disease modifying anti-rheumatic drugs; M: mean; MTX: methodrexate.
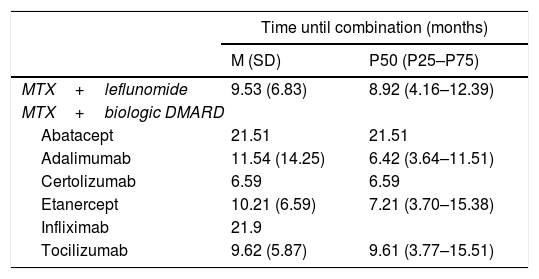
Time Elapsed Between the Start of Monotherapy With MTX and the Start of Combined Therapy of MTX + Another DMARD.
| Time until combination (months) | ||
|---|---|---|
| M (SD) | P50 (P25–P75) | |
| MTX+leflunomide | 9.53 (6.83) | 8.92 (4.16–12.39) |
| MTX+biologic DMARD | ||
| Abatacept | 21.51 | 21.51 |
| Adalimumab | 11.54 (14.25) | 6.42 (3.64–11.51) |
| Certolizumab | 6.59 | 6.59 |
| Etanercept | 10.21 (6.59) | 7.21 (3.70–15.38) |
| Infliximab | 21.9 | |
| Tocilizumab | 9.62 (5.87) | 9.61 (3.77–15.51) |
SD: standard deviation; DMARDs: disease modifying anti-rheumatic drugs; M: mean; MTX: methodrexate.
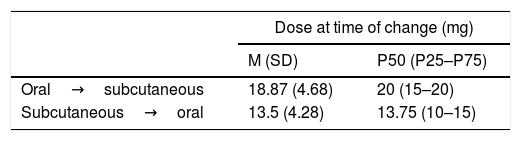
Dosing of MTX When Changes to Drug Administration Were Verified. Time Elapsed Until the Transfer Between Administration Routes.
| Dose at time of change (mg) | ||
|---|---|---|
| M (SD) | P50 (P25–P75) | |
| Oral→subcutaneous | 18.87 (4.68) | 20 (15–20) |
| Subcutaneous→oral | 13.5 (4.28) | 13.75 (10–15) |
| Time until change (months) | ||
|---|---|---|
| M (SD) | P50 (P25–P75) | |
| Oral→subcutaneous | 7.92 (7.57) | 5.70 (2.95–9.51) |
| Subcutaneous→oral | 7.71 (6.13) | 6.07 (3.7–12.98) |
SD: standard deviation; M: mean; MTX: methodrexate.
In 544 patients (97%) folic/folinic acid supplements, combined with MTX were administered. Adverse events attributed to MTX were detected in 97 peoples (17.3%). There is no available information to specify the organ/body part affected by the adverse reaction to MTX, or to quantify the intensity of them. In 97 patients (17.3%) the MTX was interrupted due to problems of pharmacological safety. Suspension was made for lack of drug efficacy in 9% of patients.
DiscussionNumerous documents of recommendations and recent clinical practice guides recommend the use of MTX as a first line DMARD for RA, based on its proven effectiveness, excellent safety profile and low cost.2–4,8,14–16 however, considerable variability is still being detected among the different rheumatologists where the initial prescription moment of the MTX is referred to, plus the initial dose, intensification protocols, interval between dose increases and administration route, as well as its combination with other drugs.5,9 This variability is apparent in this analysis, confirming the diversity of real clinical practice in the approach to the patient with RA.
In studies published on first line therapy with biologic drugs, a third of patients only treated with MTX achieved clinical remission of the RA.17,18 A recent systematic review,15 has established the clinical utility of monotherapy with MTX in RA (at a weekly dose of between 5 and 25mg, for observation periods between 12 and 52 weeks); the MTX is fairly well tolerated, with a discontinuity rate, due to adverse events, of only 16% after 52 weeks. The use of MTX is also associated with a 70% reduction in mortality in RA, mainly due to the reduction in death rates from cardiovascular events.
The conventional MTX dose in clinical trials as combined therapy is 15mg. In our study, the final mean dose of MTX in monotherapy prior to combining it with biologic DMARDs is below 17mg weekly. From 15mg, at an equal dose, the bioavailability of the MTX is always greater when administered by parenteral route.19 From this dose, and especially for the higher doses (25–30mg), a parenteral injection is recommended for greater therapeutic efficacy with the same tolerability.10,20–24 In our analysis a marked persistence in oral MTX administration was observed, since effectively the mean dose in transference of oral to subcutaneous route is almost 19mg weekly of MTX (Table 3). In the CAMERA25 study, the patients with RA who did not respond to an MTX oral dose were changed to the same subcutaneous dose, observing a clinical response after this change of administration route. A retrospective and multicentre observational study carried out in Spain confirmed how the parenteral route was used to administer higher MTX doses (above 15mg weekly) and in patients with moderate or high disease activity.26
A recently published research study which aimed to assess the use of MTX for the treatment of RA in U.S.A.27,28 confirmed that after 5 years of observation only 7% of the individuals used the parenteral route to administer MTX. The mean weekly dose after the change of route to parenteral was of 17.5±5mg, and prior to the introduction of biologic treatment was 21±5mg. In our analysis (Tables 1 and 2) the interval up until the introduction of a biologic agent oscillated between 6 and 22 months, with the MTX dose at the time of combination being somewhat lower.
In older patients more intolerance problems to MTX are detected, for different causes (impairment of renal function, hypoproteinemia)29; this could justify the lower rate of MTX prescription detected in our research in patients over 65 years of age. Supplementation with folic/folinic acid reduces the rate of adverse events and suspension of MTX without compromising the treatment efficacy.30 In our series, where over 90% of patients received supplements of folic/folinic acid, the rate of interruption of the MTX treatment for safety problems is low (15.5%).
The aim of this study has been to describe several relevant aspects on the real use of MTX in Spain, detecting possible areas of improvement for the benefit of patients. We observed that MTX is a pillar of treatment for RA although optimisation of its treatment, and particularly in what may be referred to as a dose escalation and the selection of the correct administration route depending on the dose administered, may lead to a better control of RA.
Conflict of InterestsJesús Tornero received funds for research and training from Gebro Pharma, Novartis, Pfizer and Roche.
José Luis Andreu, María Auxiliadora Martín, Héctor Corominas, José Javier Pérez Venegas y Fernando Sánchez-Alonso have no conflict of interests to declare.
José Andrés Román-Ivorra received funds for research, congress attendance, national and international presentations, and for consultation from Abbvie, Actelion, BMS, Celgene, Gebro, Janssen, Lilly, MSD, Novartis, Pfizer, Roche and UCB.
Please cite this article as: Tornero-Molina J, Andreu JL, Martín-Martínez M-A, Corominas H, Pérez Venegas JJ, Román-Ivorra JA, et al. Metotrexato en pacientes con artritis reumatoide en España: subanálisis del proyecto AR Excellence. Reumatol Clin. 2019;15:338–342.