Hepatic nodular regenerative hyperplasia (HNRH) is an entity characterized by a diffuse, benign transformation of the hepatic parenchyma into small regenerative nodules, which can lead to the development of non-cirrhotic portal hypertension (PH). It has been associated with several disorders, mainly autoimmune and hematologic diseases and immunosuppressive drugs, including azathioprine and cyclophosphamide.1 The HNRH has been described in RA/Felty's syndrome, systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), Sjögren's syndrome, systemic sclerosis, sarcoidosis, polyarteritis nodosa and mixed cryoglobulinemia.1–3 However, only one case has been reported in systemic onset juvenile idiopathic arthritis (JIA).4
A 33-year-old male was diagnosed with systemic onset JIA at 5 years of age based on fever, evanescent rash, joint pain, recurrent pericarditis, elevated acute phase reactants (C-reactive protein 22.5mg/dl and erythrocyte sedimentation rate 39mm/h) and negative for rheumatoid factor, antinuclear antibodies and HLA-B27. He was initially treated with low doses of corticosteroids and methotrexate 10mg/week, but the latter was suspended at 20 years of age due to neurotoxicity. Since then, he has received varying doses of corticosteroids alone, maintaining an active disease, with a mild increase in alanine aminotransferase (ALT) (72U/l), gamma-glutamyl transferase (GGT) (171U/l) and alkaline phosphatase (AP) (146U/l), in addition to thrombocytopenia (117×103/μl) with normal bone marrow study. Ultrasonography and abdominal computed tomography showed mild splenomegaly with no signs of PH. Tocilizumab (8mg/kg every 14 days) was initiated in January 2011, with rapid improvement of systemic symptoms and standardization of the reactants. However, after the third infusion he presented worsening liver function, doubling previous levels of ALT and GGT, increasing aspartate aminotransferase (AST) (79U/l) and total bilirubin (TB) (3.51mg/dl) levels, presenting hypofibrinogenemia (122mg/dl), abnormal INR (1.5) and an increase in the severity of thrombocytopenia (57×103/μl). He was admitted to the hospital with suspected Macrophage Activation Syndrome. The patient had not concomitantly received NSAIDs or other potentially hepatotoxic drugs. Serologic testing for hepatitis viruses B and C, Epstein Barr virus and human immunodeficiency virus were negative, and a bone marrow biopsy showed reactive changes and no hemophagocytosis. Endoscopy revealed the presence of grade i–ii esophageal varices and a biopsy confirmed the diagnosis of HNRH (Fig. 1A and B). Tocilizumab was suspended permanently, with normalization of AST, TB, fibrinogen and INR, but the levels of ALT, GGT, AP and thrombocytopenia remained unchanged. In successive imaging controls, an increased diameter of the portal vein (17mm) was observed (Fig. 1C), with no evidence of thrombosis. Currently, the patient receives only steroids at a variable dose, with mild to moderate activity of JIA and without developing other complications of PH.
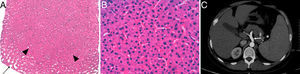
Liver biopsy in which a hyperplastic nodular zone (arrowheads) partially surrounded by dilated sinusoids (arrow) and absence of fibrosis (hematoxylin–eosin 2×) (A) is observed. At higher magnification, the sinusoids are compressed by the thickened hepatocyte plaques (hematoxylin–eosin 40×) (B). An abdominal CT scan shows dilation of the portal vein and its branches (arrow) (C).
HNRH pathogenesis is unknown, but it is considered a nonspecific tissue adaptive response to an altered distribution of hepatic vascular flow with a multifactorial cause.5 Among the identified factors which can cause this condition are thrombosis (thrombophilia, malignancies, myeloproliferative disorders, APS) and endothelial cell injury in the portal venules and hepatic sinusoids (drugs, autoimmune diseases, and viruses).1 In this case, the underlying disease and drug therapy could have been involved. The chronic inflammatory state secondary to the long duration of systemic onset JIA is associated with high circulating levels of proinflammatory cytokines such as interleukin 6 (IL-6), which has been implicated in the development of HNRH.6 This cytokine is important in the proliferation of hepatocytes and liver regeneration.7 However, transgenic mice expressing high levels of IL-6 and its soluble receptor (sIL-6R) exhibit hepatocellular hyperplastic nodules around the portal tracts, as do patients with HNRH.6 There is also clinical evidence that the increase in IL-6 is associated HNRH in Castleman's disease.8 Other HNRH related disorders, such as SLE and hematological malignancies, have high levels of the IL-6/sIL-6R complex.6 Although patients with systemic onset JIA have a higher concentration of these complexes.9 HNRH has rarely been described in this disease, with only the case of a 17-year-old patient with hepatomegaly and PH4 having been published, but this is probably due to an underdiagnosis of this condition.1 Drugs are also associated with the development of HNRH, mainly thiopurines (azathioprine, 6-mercaptopurine, 6-thioguanine), cyclophosphamide, busulfan, oxaliplatin and antiretroviral drugs.1 Our patient did not receive any of these agents but was treated for 15 years with methotrexate, a drug that has been associated with hepatotoxicity and liver fibrosis but not HNRH, with only one case found in the literature of a patient with adult-onset Still's disease treated simultaneously with azathioprine and glucocorticoids, attributing its development to the combination of the latter 2 drugs,10 and another in a patient with Felty's syndrome who was diagnosed with HNRH after receiving methotrexate for a short period of time, leading the authors to conclude that there was no association.11 This case also received varying doses of corticosteroids for 28 years. The association of these drugs with HNRH10 through an unknown mechanism has been suggested. However, chronic corticosteroid therapy is associated with the development of vascular lesions in autoimmune diseases, which could contribute to the development of liver vascular flow alterations that lead to the development of HNRH.
The possibility of HNRH should be considered in patients with symptomatic PH (splenomegaly, ascites, esophageal varices), who have normal transaminases and no manifestations of cirrhosis. HNRH is estimated to represent 27% of all cases of non-cirrhotic PH in Europe, but its diagnosis suffers a significant delay because only 50% have a PH and 11%–25% have only mildly elevated liver enzymes, generally AP.1 Our case probably developed HNRH insidiously during the prolonged course of systemic onset JIA and the emergence of hepatotoxicity during administration of tocilizumab finally led to the diagnosis, when the patient developed PH. Imaging tests have poor sensitivity and specificity for detecting HNRH, so a definitive diagnosis requires histologic confirmation.1 Treating HNRH focuses on correcting the underlying cause (autoimmune disease, hematologic disorder, drugs) and, in patients in whom PH has developed, preventing and treating the complications, with esophageal varices bleeding constituting the leading cause of mortality in these patients.1 In conclusion, HNRH is a complication that can occur during the development of autoimmune diseases, including systemic onset JIA, so it should be considered in cases with a persistent abnormal liver profile, even without other data from PH.
Please cite this article as: Sifuentes Giraldo WA, Burdaspal Moratilla A, Moreno Caparrós A, Gámir Gámir ML. Hiperplasia nodular regenerativa hepática como complicación tardía de la artritis idiopática juvenil de inicio sistémico. Reumatol Clin. 2014;10:194–195.