To describe the prevalence of comorbidities in patients with RA in Spain and discuss their management and implications using data from the Spanish cohort of the multinational study on COMOrbidities in Rheumatoid Arthritis (COMORA).
MethodsThis is a national sub-analysis of the COMORA study. We studied the demographics and disease characteristics of 200 adults patients diagnosed with RA (1987 ACR), and routine practices for screening and preventing the following selected comorbidities: cardiovascular, infections, cancer, gastrointestinal, pulmonary, osteoporosis and depression.
ResultsPatients had a mean age of 58 years and a mean RA duration of 10 years. Mean DAS28 score was 3.3 and approximately 25% of patients were in remission (DAS28 <2.6). Forty-four (22%) patients had ≥1 comorbidity, the most frequent being depression (27%) and obesity (26%). A history of myocardial infarction or stroke was observed in 5% and 1% of patients, respectively, and any solid tumor in 6%. Having a Framingham Risk Score >20% (51%), hypercholesterolemia (46%) or hypertension (41%) and smoking (25%) were the most common CV risk factors. For prostate, colon and skin cancers, only 9%, 10% and 18% of patients, respectively, were optimally monitored. Infections were also inadequately managed, with 7% and 17% of patients vaccinated against influenza and pneumococcal, respectively, as was osteoporosis, with 47% of patients supplemented with vitamin D and 56% with a bone densitometry performed.
ConclusionsIn Spain, the prevalence of comorbidities and CV risk factors in RA patients with established and advanced disease is relatively high, and their management in clinical daily practice remains suboptimal.
Describir la prevalencia de comorbilidades en pacientes con AR en España y discutir sobre su manejo en la clínica diaria utilizando los datos de la cohorte española del estudio internacional COMORA.
MétodosSubanálisis nacional del estudio COMORA en el que se analizaron las características demográficas y clínicas de 200 pacientes con AR (1987 ACR) y las prácticas rutinarias para el cribado y la prevención de eventos cardiovasculares (CV), gastrointestinales y pulmonares, infecciones, cáncer, osteoporosis y depresión.
ResultadosLos pacientes tenían una edad media de 58 años, una duración media de la enfermedad de 10 años, un DAS28 de 3,3 y el 25% estaba en remisión (DAS28 <2,6). El 22% de los pacientes presentaba al menos una comorbilidad, principalmente depresión (27%) y obesidad (26%). El 5% tenía historia de infarto de miocardio, el 1% de ictus y el 6% de tumor sólido. Una puntuación de Framingham >20% (51%), tener hipercolesterolemia (46%), hipertensión (41%) y fumar (25%) fueron los factores de riesgo CV más comunes. En relación con el cáncer de próstata, colon y piel, solo el 9, 10 y el 18% de los pacientes, respectivamente, estaban óptimamente controlados. Las infecciones tampoco se manejaban de forma óptima, con solo el 7 y el 17% de los pacientes vacunados contra la influenza y neumococo, respectivamente, al igual que la osteoporosis, con el 47% suplementados con la vitamina D y el 56% con una densitometría realizada.
ConclusionesEn España, la prevalencia de comorbilidades y factores de riesgo CV en pacientes con AR establecida y avanzada es relativamente alta, y su manejo en la clínica diaria continúa siendo subóptimo.
Rheumatoid arthritis (RA) is a chronic inflammatory disease that can lead to progressive joint deformity, disability and reduction of life expectancy. In addition to the consequences of the disease itself, patients with RA are at increased risk of developing comorbidities that contribute substantially to increased disability and worsened quality of life. Cardiovascular, lung and gastrointestinal diseases, malignancies, infections, psychiatric disorders, and osteoporosis are found to be more prevalent in patients with RA compared to the general population, mainly due to concomitant medication such as immunosuppressive drugs, immunomodulatory effects of the disease, and increased prevalence of cardiovascular (CV) risk factors.1,2
The prognosis of RA has improved over years because of the considerable change in the treatment, which includes tight control, aiming for low disease activity or remission. However, comorbidities in RA are often underrecognized despite their impact on disease activity and treatment outcomes3, and the awareness of most rheumatologists about their responsibility in their management.4 Several studies, such as the international COMORA on prevalence of comorbidities in RA and their monitoring, reported that compliance with guidelines on how to detect and manage these comorbidities is far from optimal, and varies between countries.5 To address this issue, EULAR has just published recommendations for comorbidity risk management in patients with RA,4 which are expected to be easily implemented in daily practice and will be of interest to evaluate in future studies. In Spain, the Society of Rheumatology of the Community of Madrid (SORCOM) has also developed practical recommendations to guide rheumatologists on optimal diagnosis and management.6
How Spain could better follow and implement such guidelines will be determined by the findings of the present study by showing the gap between recommendations and routine practices existing in 2012 in the management of RA comorbidities.
Using data from the Spanish cohort of COMORA study, we describe the prevalence of comorbidities in patients with RA in Spain and discuss their management and implications in clinical practice. The rationale for a national sub-analysis of COMORA was the need to emphasize the mistakes that rheumatologists do in identifying and monitoring these conditions in RA and clarify the role they should have in this area.
MethodsStudy Design and PatientsCOMORA was a cross-sectional, observational, multicenter, international study to evaluate variability in the prevalence of comorbidities and their risk factors between participating countries.5
The study was conducted according to the ethical principles of the Declaration of Helsinki and was approved by all local ethics review committees, with written informed consent obtained from all patients.
Eligible patients in COMORA were at least 18 years old, fulfilled the 1987 American College of Rheumatology classification criteria for RA, and were able to understand and complete the administered questionnaires.
A total of 20 centers in Spain included patients in the COMORA study with an expected enrolment of at least 200 patients. Sample size of the COMORA has been previously reported.5
Data CollectedData collection in COMORA5 is briefly described below. For each patient, information was gathered by a study investigator during a face-to-face interview at a dedicated study visit and through review of the medical record.
Demographics and disease-related variables. Information was collected on patient age, gender, body mass index (BMI), smoking habit, alcohol intake, marital and socioeconomic status, and educational level. Disease activity was measured by DAS28 based on the erythrocyte sedimentation rate (ESR) and by C-reactive protein (CRP) levels. Disease severity was assessed using the history of joint surgery to address structural damage caused by RA. Medications for RA (previous or current) including nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids and conventional and biological disease-modifying antirheumatic drugs (DMARDs) were also collected.
Past or current comorbidities. The comorbidities evaluated were selected for the study by the scientific committee and were not all-inclusive: myocardial infarction or stroke, cancers (prostate, breast, uterus, colon, cutaneous melanoma, basal cell carcinoma of skin, lung) and lymphoma, gastrointestinal (diverticulitis, ulcers), hepatitis, pulmonary diseases (chronic obstructive pulmonary disease (COPD), asthma), and depression.
Coexisting risk factors. Traditional CV risk factors (hypertension, diabetes, dyslipidemia, family history of myocardial infarction or sudden death) were collected along with risk factors for infections and cancers.
Management of RA-associated comorbidities. Management practices were assessed based on other international cohorts,7,8 and recommendations of the French Society of Rheumatology and the French Ministry of Health in force at the time of COMORA.
Statistical AnalysisDescriptive statistics were used for baseline characteristics, prevalence of comorbidities and associated risk factors, with 95% confidence intervals (CI). The percentage of patients optimally screened for these comorbidities and managed according to national guidelines was calculated. To examine how the variables age, disease duration, HAQ (health assessment questionnaire) and DAS28 scores, and treatment with biologics affected the number of comorbidities, multivariate analysis was conducted.
Compliance with recommendations was analyzed according to the COMORA study.5 Briefly, CV risk assessment should be annual and patients older than 50 years with prior history of CV events or a Framingham risk score >20% (depending of the country) should have received any antithrombotic agent. Thresholds used were blood pressure (BP) >140/90mmHg for hypertension (except for patients with diabetes where it was >130/70mmHg), LDL cholesterol>the targeted value defined with regard to the number of additional CV risk factors for hypercholesterolemia, and blood glucose (BG) >1.26 for hyperglycemia. Screening procedures for prostate, breast, uterine, colon, skin and lung cancers included digital rectal examination, prostate-specific antigen (PSA) levels, mammography, Papanicolau (Pap) smear, fecal occult blood test, colonoscopy, visit to dermatologist, and chest X-ray. Procedures for malignancy risk factors included family history of prostate or colon cancer and personal history of inflammatory bowel disease. Infections were optimally managed with a dental examination and influenza vaccination in the previous year, and a pneumococcal vaccination in the previous 5 years. The optimal screening for osteoporosis included a bone densitometry and current supplementation with vitamin D.
ResultsPatient CharacteristicsPatient characteristics are shown in Table 1. Mean age was 58.5 years and mean disease duration was 10.3 years. Disease activity was low, as shown by the mean DAS28 of 3.3, and approximately 25% of patients were in remission (DAS28 <2.6). At the time of the study, 94.5% of patients were taking DMARDs and 36.5% biological agents.
Patient Baseline Characteristics.
| Variable | No.=200 |
|---|---|
| Age (years), mean (SD) | 58.5 (12.4) |
| Female, n (%) | 158 (79.0) |
| Smoking status (current smokers), n (%) | 17 (8.5) |
| Educational level (university or secondary school), n (%) | 93 (46.5) |
| Overweight, n (%) | 118 (59.0) |
| Work status (currently employed), n (%) | 70 (35.0) |
| Disease duration (years), mean (SD) | 10.3 (8.3) |
| DAS28-ESR, mean (SD) | 3.3 (1.4) |
| HAQ, mean (SD) | 1.1 (0.7) |
| CRP (mg/dL), mean (SD) | 3.9 (13.8) |
| At least one comorbidity, n (%) | 44 (22.0) |
| Prednisone (currently taking), n (%) | 105 (52.5) |
| NSAIDs use within the previous 3 months), n (%) | 112 (56.0) |
| DMARDs (ever treated), n (%) | 199 (99.5) |
| Number of prior DMARDs, mean (SD) | 2.8 (1.6) |
| DMARDs (currently taking), n (%) | 189 (94.5) |
| MTX (ever treated), n (%) | 193 (96.5) |
| MTX (currently taking), n (%) | 149 (74.5) |
| Any biological therapy (ever treated), n (%) | 86 (43.0) |
| Any biological therapy (currently taking), n (%) | 73 (36.5) |
| Anti-TNF (ever treated), n (%) | 74 (37.0) |
| Tocilizumab (ever treated), n (%) | 15 (7.5%) |
Abbreviations: CRP, C-reactive protein; DAS28-ESR, disease activity score 28-erythrocyte sedimentation rate; HAQ, health assessment questionnaire; MTX, methotrexate; SD, standard deviation; TNF, tumor necrosis factor.
Of the 200 patients analyzed in the Spanish cohort, 44 (22%) had at least one comorbidity. This prevalence rate resulted from the exclusion of depression from the analysis since it was not formally diagnosed. These patients were significantly older (63.0 vs 57.2 years; P=.005), had worse HAQ scores (1.3 vs 1.0; P=.02), and exhibited higher CRP levels (6.8 vs 3.1; P=.01) than those without comorbidities. Patient age was the only factor independently associated with the presence of comorbidities (OR 1.038, 95% CI 1.007–1.069; P=.014).
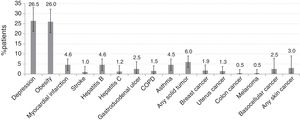
Fig. 1 shows the prevalence of comorbidities among RA Spanish patients. The most frequent were depression (past or current) (54 patients [27.0%, 95% CI 20.5%–33.2%]) and obesity (52 patients [26.0%, 95% CI 20.2%–32.8%]). A history of myocardial infarction or stroke was observed in 10 patients (5.0%, 95% CI 2.1%–8.5%) and 2 patients (1.0%, 95% CI 0.1%–3.7%), respectively, and any solid tumor in 12 patients (6.0%, CI 95% 3.1%–10.3%).
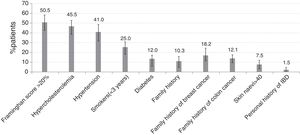
Regarding risk factors for comorbidities (Fig. 2), the most frequent were those for CV risk: Framingham risk score >20% (102 patients [51.0%, 95% CI 43.4%–57.6%]), hypercholesterolemia (92 patients [46.0%, 95% CI 38.5%–52.7%]), hypertension (82 patients [41.0%, 95% CI 34.1%–48.2%]), obesity (52 patients [26.0%, 95% CI 20.2%–32.8%]), tobacco use within previous 3 years (50 patients [25.0%, 95% CI 19.2%–31.6%]), and diabetes (24 patients [12.0%, 95% CI 7.8%–17.3%]).
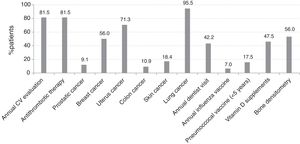
Screening Procedures for Comorbidities and Risk FactorsCardiovascular DiseasesAnnual CV risk assessment, including BP measurement, total serum cholesterol (HDL and LDL), BG and serum creatinine, was performed in 81.5% of the patients. Among 11 patients with prior myocardial infarction or stroke, 5 (45.5%) were not currently receiving antithrombotic therapy. Thirty-two patients of 189 without a history of myocardial infarction or stroke, but older than 50 years and with a Framingham risk score >20% were not under antithrombotic therapy. Thus, 32 (18.5%) patients were not being optimally managed to prevent cardiovascular events (Fig. 3). Among 131 patients without hypertension, 34 (26%) had elevated BP. Of 180 patients without diabetes, 4 (2.2%) had increased BG.
InfectionsDuring the year before the study, 42.2% of patients had undergone a dental examination. Influenza and pneumococcal vaccines were given to 7.0% and 17.5% of patients within the previous year and the last 5 years, respectively (Fig. 3). In only 2.1% of patients were both influenza and pneumococcal vaccination performed according to current recommendations. The percentage of patients on biological agents vaccinated against Streptococcus pneumoniae was 30.1% and 8.2% against influenza.
CancersAmong males aged 45–50 years without known prostate cancer, a digital rectal examination and PSA test had been performed in 58.3% and 27.8%, respectively. For breast cancer screening, 74.8% of women between the ages of 50 and 74 years without known breast cancer had a mammogram within the previous 2 years. For colon cancer, 21.8% and 14.5% of patients over 50 years old without known colon cancer underwent any colonoscopy and a fecal occult blood test, respectively. For uterine cancer, 64.3% of women aged 25–65 years had been screened within the previous 3 years with Pap smear. The rate of chest X-ray for lung cancer was 95.5%. For skin cancer, 19.1% of patients underwent a dermatological consultation, which was annually in the case of patients with more than 40 nevi.
OsteoporosisFrom diagnosis of RA, bone densitometry was performed in 36.6% of patients younger than 50 years and in 58.6% of those aged 50 years and older. Current vitamin D supplementation, however, was more frequent in patients younger than 50 years (93.3%) than in those aged 50 years and above (75%). Over half of patients (55.2%) were supplemented with vitamin D during prednisone treatment.
DiscussionThis study shows that prevalence of comorbidities and CV risk factors in patients with established and advanced RA is relatively high in Spain, and evaluation of their monitoring is suboptimal despite being a country with an effective and accessible health system. The rate of 22% of at least one comorbidity found is lower as in other studies,9 but precisely this possible underestimation reflects their inadequate screening in RA. Indeed, if adequate screening for depression had been included in the analysis, this percentage may have actually been higher. The good control of disease that the Spanish cohort had at the time of the study may also have contributed to fewer rheumatology visits and, therefore, to inadequate systematic evaluation and monitoring of risk factors for comorbidities. The role of rheumatologists in the management of comorbidities is not clearly defined, and EULAR have published recommendations for their screening and prevention in chronic inflammatory rheumatic diseases.4 We discuss how Spain could better implement such guidelines with the help of a consensus framework for the management of comorbidity in RA. To begin with, the clinical history, physical examination and electronic information need to be optimized.6
Depression was the most frequent comorbidity reported in the Spanish cohort in 26% of patients, coherent with the high prevalence in RA which may reach 42%.10 This rate, however, vary among countries5 and also according to the screening tool.11 In COMORA, depression was self-reported instead of using a validated scale and was finally excluded from the analysis. It would have been too ambitious to expect an appropriate management from a misdiagnosis, especially when the rheumatologists’ responsibility in this regard is not clear. In Spain, their role is limited to observing, looking for feelings of depression through annual patient history examination to ensure appropriate referral.6
The prevalence of obesity in the Spanish cohort (26%) is comparable to another national study,12 but well above than in general Spanish population (16% in men and 18% in women).13 Obesity was not addressed in COMORA and, consequently, we cannot conclude whether strategies for its screening and prevention in Spain are optimally implemented. Nonetheless, from this prevalence we can infer that obesity remains uncontrolled and that patients do not receive medical advice on health promotion despite it being recommended in rheumatology practices along with an annual physical examination.6 Efforts are needed to ensure that health professionals caring for RA patients understand the importance of obesity and their competences, especially given the globally increasing prevalence and the rise in incidence of RA attributed to it.14
The 6% prevalence of CV events in Spain agrees with the latest national report in the general population.15 However, the trend to an increased CV risk in RA is confirmed in our cohort, with over 40% of patients with hypertension and hypercholesterolemia. Almost all patients received an annual CV risk evaluation following EULAR recommendations,16 and also coinciding with the minimal work-up should be done by rheumatologists, who must act as observers and advisors.6 On the other hand, CV risk should be assessed according to national guidelines as they may differ among countries. In COMORA, the Framingham model was used instead of the calibrated Framingham-REGICOR function adapted to the Spanish population, which assigns a lower coronary risk category.17 Hence, the classical Framingham equation had probably overestimated CV risk in the Spanish cohort. Nevertheless, a high percentage of patients were receiving antithrombotic therapy. The excess CV risk despite treatment might be explained by the presence of additional risk factors such as obesity and smoking in around a quarter of patients, two of the main extrinsic factors for RA and also for individualized CV risk, which do not seem to be targets in Spanish daily practice. Unfortunately, information concerning lifestyle medical advice was not available. In this regard, rheumatologists are advised to help their patients to quit smoking or change their diet using best-evidence based methods.16
RA patients in Spain are also regularly screened for prostate, breast, colon, cervix, and skin cancers, given the percentages of patients who never underwent a PSA test (41%), mammography for over two years (25%), colonoscopy (78%), Pap smear for over three years (36%) and never were referred to a dermatologist (81%), respectively. These results are somewhat comparable to previous studies that suggested an inadequate quality of care in RA patients,18 but also to others that showed similar cancer screening rates in patients with and without RA.5,19 This is why we cannot affirm that this suboptimal management is specific to RA, and neither can we expect that French recommendations for screening and prevention of malignancies used in COMORA are fully adapted to our local level. Far behind EULAR principles,4 screening procedures for breast and colon cancer risk factors were documented in only 10% and 18% of patients, respectively. The advanced age is associated with enhanced comorbidity and in our study, it seems to be the only predictive factor. If one third of RA patients are diagnosed at 60 years or older, the question is to what extent RA contributes to the risk for some comorbid illnesses other than explained by disease activity or its treatment.
Whereas management of osteoporosis seems to have improved over the years,20 it remains suboptimal in Spain since last report21 even though half of patients were under prednisone and around menopausal age, two factors influencing osteoporosis risk in RA,20 and despite the responsibility of rheumatologists in decision-making and coordinating specialty referrals.6 The limited or no access to densitometry in several centers may have been responsible for the observed suboptimal bone mineral density testing. The high use of vitamin D among patients who had a bone densitometry would indicate the intention to prevent further bone loss, if not for the fact that 45% of corticosteroids users were not given prophylaxis, which in theory must be offered to all patients. These results could be explained in part by differences in prescribing patterns of antiosteoporotic drugs across Spain.22 Calcium supplementation as part of preventative therapies, particularly in patients receiving steroids and pharmacological interventions such as bisphosphonates, was not investigated in COMORA and, consequently, nor in the Spanish cohort.
As in other studies,23,24 our patients were not adequately covered for influenza and pneumococcal immunization despite the susceptibility to infections due to comorbidities and treatment with corticosteroids and DMARDs.25 Nonetheless, the need for a greater immunization in patients on biological therapy is coherent with the high risk of infections related to biologics26 and the effectiveness of those vaccinations against some agents.27 In fact, the Spanish Society of Rheumatologist (SER) recommends vaccinating 100% of people treated with biological therapies.28 Further, patients receiving major immunosuppressants including biologics are less likely to be immunized for pneumococcal disease,29 which does not agree with the high rate that in our cohort were offered pneumococcal and no influenza vaccination. We do not believe that evidence of diminished or impaired immune responses to vaccines following immunomodulators had influenced this trend, especially when influenza vaccination is warranted to all RA patients regardless of treatment.30 Although assisted by infectious disease specialists, rheumatologists should be in charge of serious infections by checking symptoms, vaccinations, and laboratory tests for CRP at every visit.6
The possible biases in a prevalent cohort study of a chronic disease highlighted in COMORA, may have affected the estimation of some comorbidities. Other criticism is the small number of patients that could compromise the generalization of results. However, the consecutive inclusion of unselected patients from rheumatologist units across Spain in an observational setting ensures the representativeness of our sample. In fact, demographics and disease characteristics of this cohort mirror those from others indicating this sample reflects the general RA population.
In conclusion, findings from the Spanish cohort of COMORA confirm what growing evidence shows about poor control of comorbidities in RA and associated risk factors in daily practice. There are, however, several shortcomings in the design of the global COMORA that encourage considering the possibility that these results do not fully reflect practice in Spain. Regardless of this, efforts are need to reinforce health professionals’ awareness of the importance of a comprehensive approach to comorbidities as part of the care of patients with RA.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Source of FundingThis study was conducted with the support of an unrestricted grant from Roche Ltd.
Conflict of InterestsA.B. has received research grants from Roche, MSD, Pfizer, Abbvie, BMS and UCB. He is consultant and a member of speakers’ bureaus for Pfizer and has received speaker honoraria from Pfizer, Roche, MSD, Pfizer, Abbvie, BMS and UCB. L.L.O. has received grant for Roche and payment for lectures from Roche and Abbvie. M.A.L. has received payment for consultancy, lectures and board membership from Roche, Abbvie, BMS and MSD. C.O.C. has received grant from Roche and payment for lectures from UCB. L.P.P. has received consulting fee or honorarium. A.C. has received grant form Roche. J.R.R. has received fees for participation in review activities from Roche and payment for lectures from Roche, Abbvie and BMS. S.O. has received grant from Roche. S.M.F. has received payment for consultancy from Roche. J.M.N. has received fees for participation in review activities, and payment for consultancy and lectures from MSD, UCB, Gebro, Amgen, Roche, BMS and Abbvie. R.G.V. has received grant from Roche and payment for consultancy from Actelion, BMS, Pfizer, Hospira, Janssen, Sandoz, UCB and Roche, payment for expert testimony from BMS, UCB, the Spanish Society of Rheumatology and grants/grants pending from MSD, Abbvie and Roche. B.H.C. has received grant from Fundación Pública Andaluza para la Gestión de la Investigación Pública de Sevilla, and support for travel to meetings from EULAR 2015. She is receiving payment for consultancy, lectures and board membership from Roche, Lilly, Bristol, Amgen, MSD, the Spanish Society of Rheumatology and Gebro Pharma, and grants/grants pending from Roche and the Andalusian Society of Rheumatology. J.C. has received grant from Roche. J.R.G. has received grant from Roche. J.F.G.L. has received grant from BIOEF and Osakidetza, payment for consultancy and lectures from Pfizer, Rovi, Abbvie, UCB, Roche, MSD, BMS and Novartis, and travel/accommodations meeting expenses unrelated to activities described from MSD, Abbvie, Pfizer, Roche, BMS, UCB, Janssen and Novartis. A.G.C. has received grant from Roche, and payment for consultancy, lectures and development of educational presentations from Roche, Pfizer, MSD, Abbvie, Sanofi, Rubió and Lilly, and meeting expenses from Roche and Pfizer. R.S. has received grant from Roche. L.A.A. has received grant from Roche. A.F.N. has received grant from Roche and payment for consultancy and lectures from Pfizer, Roche and Celgene. L.R.R. has received grant from Roche. M.A.G.G. has received payment for consultancy from Lilly and lectures from Abbvie, Roche, Bristol and Pfizer. E.M.M. has received grant from Roche and payment for consultancy, lectures and board membership from Sanofi, MSD, Pfizer, Roche, Biogen and Hospira. F.G., and I.H. did not declare the competing interests and the rest of the authors declare not to have any conflict of interest.
The authors would like to acknowledge the patients and doctors who participated in the study and the COMORA Committee for their support, especially Dr. Dougados, who was the principal investigator for the study. Medical writing assistance was provided by Isabel Caballero at Dynamic Science S.L. during the preparation of this article. Responsibility for opinions, conclusions and interpretation of data lies with the authors.