The main purpose of this recommendation statement is to provide clinicians with the best available evidence and the best opinion agreed upon by the panellists for a rational use of synthetic disease modifying antirheumatic drugs (DMARDs) and biologicals in psoriatic arthritis (PsA) patients. The present document also focuses on important aspects in the management of PsA, such as early diagnosis, therapeutic objectives, comorbidities and optimisation of treatment.
MethodsThe recommendations were agreed by consensus by a panel of 8 expert rheumatologists, previously selected by the Spanish Society of Rheumatology (SER) through an open call. The phases of the work were: identification of key areas for updating the previous consensus, analysis and synthesis of scientific evidence (modified Oxford system, Centre for Evidence-based Medicine, 2009) and formulation of recommendations based on this evidence and by consensus techniques.
ResultsSeventeen recommendations were issued for the treatment of PsA patients. Six of them were of general nature, ranging from the early diagnosis and treatment to the importance of assessing comorbidities. The other 11 were focused on the indications for DMARDs and biological therapy in the distinct clinical forms of the disease. Likewise, the situation of failure of the first biological is addressed and treatment algorithms and a table with the different biological therapies are also included.
ConclusionsWe present the update of SER recommendations for the treatment of PsA with DMARDs and biologics.
La primera finalidad de este documento de recomendaciones es proporcionar al clínico la mejor evidencia disponible y, en su defecto, la mejor opinión consensuada por los panelistas para un uso racional y fundado de las diversas opciones de tratamiento con fármacos antirreumáticos modificadores de la enfermedad (FAME) sintéticos y biológicos en artropatía psoriásica (APs). El presente documento también incide sobre aspectos importantes en el manejo de la APs, como el diagnóstico precoz, los objetivos terapéuticos, las comorbilidades y la optimización del tratamiento.
MétodosLas recomendaciones se consensuaron a través de un panel de 8 reumatólogos expertos, previamente seleccionados por la Sociedad Española de Reumatología (SER) mediante una convocatoria abierta. Las fases del trabajo fueron: identificación de las áreas claves para la actualización del consenso anterior, análisis y síntesis de la evidencia científica (sistema modificado de Oxford, CEBM, 2009) y formulación de recomendaciones a partir de esta evidencia y de técnicas de consenso.
ResultadosSe emiten un total de 17 recomendaciones para el tratamiento de los pacientes con APs. Seis de ellas de carácter general, que abarcan desde la transcendencia del diagnóstico y tratamiento precoz hasta la importancia de las comorbilidades. El resto, las 11 específicas, se centran en las indicaciones de los FAME y la terapia biológica en las diferentes formas clínicas de la enfermedad. Así mismo, se abordan las situaciones de fracaso a un primer biológico y se incluyen los algoritmos de tratamientos y una tabla con las diferentes terapias biológicas.
ConclusionesSe presenta la actualización de las recomendaciones de la SER para el tratamiento de la APs con FAME y terapia biológica.
Psoriatic arthritis (PsA) is a heterogeneous disease due its diverse musculo-skeletal phenotypes (peripheral arthritis, axial disease, enthesitis, dactylitis), and its extra-articular manifestations, in particular the skin and nails, as well as other organs (ileitis, inflammatory bowel disease).1,2 Although skin psoriasis and PsA share certain pathophysiological processes like angiogenesis and increased proinflammatory cytokine expression, there are differences such as the unequal efficacy of certain drugs for the skin and the joints.3,4
The conventional synthetic disease-modifying antirheumatic drugs (cDMARDs) were first tried on rheumatoid arthritis (RA), and in general there is limited evidence for their efficacy in PsA.5,6 On the other hand, the inclusion of the biological therapies, TNF-alpha inhibitors, has fundamentally improved management of this disease. Nevertheless, there are a great many patients with PsA for whom these therapies are contraindicated, lose their efficacy or have side effects.
Fortunately, PsA has gone from a disease whose treatment was derived from that of RA, to become a priority disease for the research and development of new therapeutic targets. The modulating biological therapies that target IL23/IL17 (ustekinumab, secukinumab), and targeted synthetic disease-modifying antirheumatic drugs (tDMARDS) (apremilast) are now a reality for our patients.7 Although they have all demonstrated efficacy and safety in treating PsA, due to the lack of clinical trials where they have been directly compared (head-to-head studies), and recorded clinical experience, there are no clear recommendations as to the order, stage or domain of the disease when the different available drugs should be given.8
In the management of PsA it is also very important to define the treatment objective properly, and to use certain treatment strategies (tight control).
For all the above reasons, recommendations are required based on the most recent evidence, and on expert opinion on PsA treatment. EULAR9 and GRAPPA10 have recently revised their 2011 and 2009 recommendations, respectively. In this paper we present the updated recommendations of the Spanish Society of Rheumatology (SER),11 which seek to help rheumatologists to optimise the therapeutic management of PsA. This document not only gathers the principal aspects of control and treatment with biological drugs but also covers important aspects in the management of PsA such as early diagnosis, therapeutic objectives, the use of synthetic DMARDs, comorbidities, and treatment optimisation. However, the recommendations focus chiefly on therapeutic strategies using synthetic and biological DMARDs. Under no circumstances do these recommendations claim to be a strict protocol for the management and treatment of the disease, but rather to serve as a basis to increase the quality of care of patients with PsA, and help therapeutic decision-making.
Material and MethodsWe used a qualitative synthesis of the scientific evidence and consensus techniques in this project (“reasoned judgement” and “modified Delphi technique”), agreed by experts based on their clinical experience, and the scientific evidence.
Phases of the ProcessA series of steps were followed in preparing the recommendations document as described below:
Creation of the working group. The paper was started by setting up a panel of experts selected through an open call to all SER members. The Committee for Practice Guidelines (CPG) and SER Recommendations evaluated the curricula of the applicants according to objective criteria of their contribution to knowledge on PsA, principally by participation in journal publications of impact over the past 5 years. The panel of experts comprised 8 rheumatologists, members of SER. The clinical and methodological aspects were managed by one of these rheumatologists as the principal investigator (PI), and by a methodology specialist, SER Investigation Unit (IU) technician.
Identification of the key areas for updating the previous consensus. All the members of the working team participated to structure the document, and establish the content and key aspects. The recommendations from the previous Consensus and the last version of the 2015 ESPOGUIA12 were chosen for updating. First, the clinical questions were identified that might most impact the use of biological therapy for PsA. Then the content and results that did not have to answer the formulation of the research question were established. The methodology to follow in the process of preparing the recommendations was also defined.
Literature search. The clinical questions were reformulated in 7 questions in PICO format. A search strategy was designed to answer the questions, and the scientific evidence from studies published up to February 2016 was reviewed. The databases used were: PubMed (MEDLINE), EMBASE, and Cochrane Library (Wiley Online). The process was completed with a manual search of references, posters and conference summaries that the reviewers and experts considered of interest. The literature search strategies, from the 7 systematic reviews (SR), can be found in the supplementary material that is detailed in a methodological annex on the SER website.
Analysis and synthesis of the scientific evidence. Various rheumatologists, from the SER working group of evidence reviewers, were in charge of systematically reviewing the available scientific evidence. After a critical reading of the full text of the studies chosen for each review, they prepared an abstract using a standardised form including tables and text to describe the methodology, results and quality of each study. The reasons for excluding the articles that were not included in the selection were detailed. The overall level of scientific evidence was assessed using the modified levels of evidence of the Oxford Centre for Evidence-based Medicine (CEBM) (http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009).
Formulation of the recommendations. When the critical reading had been completed, the PI and the expert panel went on to formulate specific scientific evidence-based recommendations. This formulation was based on “formal assessment” or “reasoned judgement”, summarising the evidence beforehand for each of the clinical questions. The quality, quantity and consistency of the scientific evidence were considered, commonality of the results, their applicability and clinical impact. Two consensus rounds were used to formulate the recommendations; first, in an in-person meeting, with the “reasoned judgement” consensus system, all the experts drafted and discussed the recommendations and they were discussed in the presence of the methodologist; then, using a Delphi questionnaire, the level of agreement of the experts was agreed scoring each of the recommendations using a Likert scale from 1 to 5 (1: completely disagree; 2: moderately disagree; 3: neither agree nor disagree; 4: moderately agree; 5: completely agree). A high level of agreement was defined for drafting when more than 75% panellists awarded ≥4 on the Likert scale. The level of evidence and grading of the strength of recommendations were based on the modified Oxford system 2009.
Public exposure. The draft of this SER Recommendations document underwent a process of public exposure by partner members of SER and of different interest groups (pharmaceutical industry, other scientific societies, and patient associations), to determine their assessment and scientific argument for the methodology and the recommendations.
StructureThe document gathers all the recommendations, formulated and subdivided into two sections: general principles and specific recommendations. A therapeutic algorithm was created based on the recommendations, which presents the treatment approach after diagnosis of PsA in summarised form.
ResultsA total of 17 recommendations were formulated on the treatment and use of biological therapies for PsA (Tables 1 and 2).
SER Recommendations on the Treatment and Use of Biological Therapies for Psoriatic Arthritis. General Recommendations.
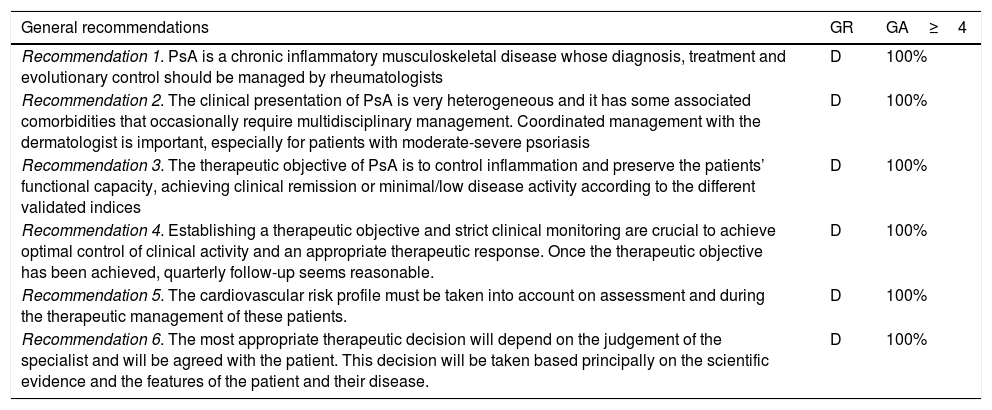
| General recommendations | GR | GA≥4 |
|---|---|---|
| Recommendation 1. PsA is a chronic inflammatory musculoskeletal disease whose diagnosis, treatment and evolutionary control should be managed by rheumatologists | D | 100% |
| Recommendation 2. The clinical presentation of PsA is very heterogeneous and it has some associated comorbidities that occasionally require multidisciplinary management. Coordinated management with the dermatologist is important, especially for patients with moderate-severe psoriasis | D | 100% |
| Recommendation 3. The therapeutic objective of PsA is to control inflammation and preserve the patients’ functional capacity, achieving clinical remission or minimal/low disease activity according to the different validated indices | D | 100% |
| Recommendation 4. Establishing a therapeutic objective and strict clinical monitoring are crucial to achieve optimal control of clinical activity and an appropriate therapeutic response. Once the therapeutic objective has been achieved, quarterly follow-up seems reasonable. | D | 100% |
| Recommendation 5. The cardiovascular risk profile must be taken into account on assessment and during the therapeutic management of these patients. | D | 100% |
| Recommendation 6. The most appropriate therapeutic decision will depend on the judgement of the specialist and will be agreed with the patient. This decision will be taken based principally on the scientific evidence and the features of the patient and their disease. | D | 100% |
GA: grade of agreement; GR: grade of recommendation; SER: Spanish Rheumatology Society.
SER Recommendations on the Treatment and Use of Biological Therapies in Psoriatic Arthritis. Specific Recommendations.
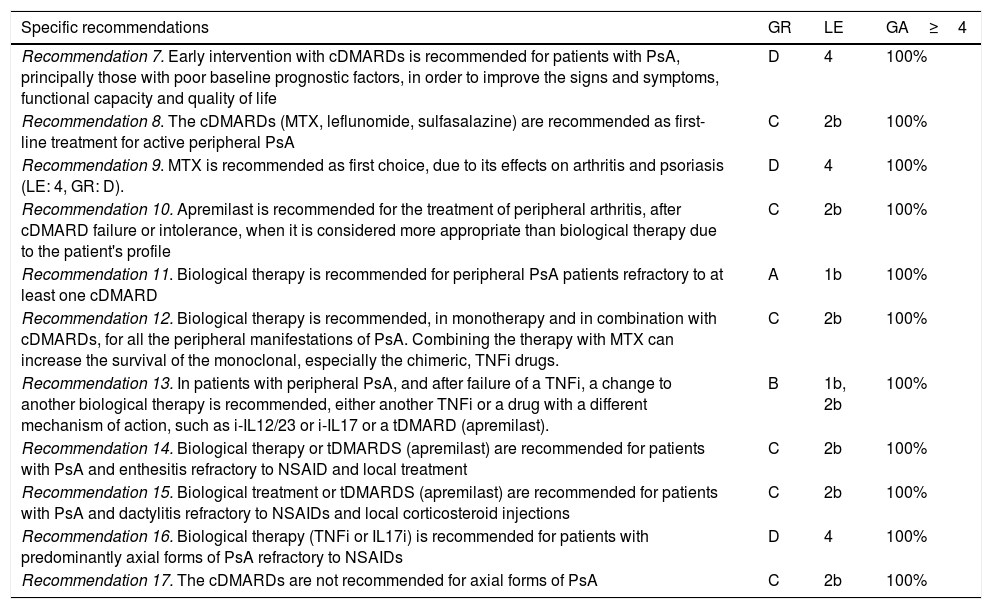
| Specific recommendations | GR | LE | GA≥4 |
|---|---|---|---|
| Recommendation 7. Early intervention with cDMARDs is recommended for patients with PsA, principally those with poor baseline prognostic factors, in order to improve the signs and symptoms, functional capacity and quality of life | D | 4 | 100% |
| Recommendation 8. The cDMARDs (MTX, leflunomide, sulfasalazine) are recommended as first-line treatment for active peripheral PsA | C | 2b | 100% |
| Recommendation 9. MTX is recommended as first choice, due to its effects on arthritis and psoriasis (LE: 4, GR: D). | D | 4 | 100% |
| Recommendation 10. Apremilast is recommended for the treatment of peripheral arthritis, after cDMARD failure or intolerance, when it is considered more appropriate than biological therapy due to the patient's profile | C | 2b | 100% |
| Recommendation 11. Biological therapy is recommended for peripheral PsA patients refractory to at least one cDMARD | A | 1b | 100% |
| Recommendation 12. Biological therapy is recommended, in monotherapy and in combination with cDMARDs, for all the peripheral manifestations of PsA. Combining the therapy with MTX can increase the survival of the monoclonal, especially the chimeric, TNFi drugs. | C | 2b | 100% |
| Recommendation 13. In patients with peripheral PsA, and after failure of a TNFi, a change to another biological therapy is recommended, either another TNFi or a drug with a different mechanism of action, such as i-IL12/23 or i-IL17 or a tDMARD (apremilast). | B | 1b, 2b | 100% |
| Recommendation 14. Biological therapy or tDMARDS (apremilast) are recommended for patients with PsA and enthesitis refractory to NSAID and local treatment | C | 2b | 100% |
| Recommendation 15. Biological treatment or tDMARDS (apremilast) are recommended for patients with PsA and dactylitis refractory to NSAIDs and local corticosteroid injections | C | 2b | 100% |
| Recommendation 16. Biological therapy (TNFi or IL17i) is recommended for patients with predominantly axial forms of PsA refractory to NSAIDs | D | 4 | 100% |
| Recommendation 17. The cDMARDs are not recommended for axial forms of PsA | C | 2b | 100% |
NSAIDs: Non-steroidal anti-inflammatory drugs; DMARDs: disease-modifying antirheumatic drugs; cDMARDs: conventional synthetic DMARDs; tDMARDs: targeted DMARDs; GA: grade of agreement; GR: grade of recommendation; IL12i, IL23i or IL17i: interleukin inhibitor 12, 23 or 17; PDE4i: TNFi: tumour necrosis factor inhibitors; LE: level of evidence; SER: Spanish Society of Rheumatology.
- •
Recommendation 1. PsA is a chronic inflammatory musculoskeletal disease whose diagnosis, treatment and evolutionary control should be managed by rheumatologists.
- •
Recommendation 2. The clinical presentation of PsA is very heterogeneous, and it has some associated comorbidities that occasionally require multidisciplinary management. Coordinated management with the dermatologist is important, especially for patients with moderate-severe psoriasis.
- •
Recommendation 3. The therapeutic objective of PsA is to control inflammation and preserve the patients’ functional capacity, achieving clinical remission or minimal/low disease activity according to the different validated indices.
- •
Recommendation 4. Establishing a therapeutic objective and strict clinical monitoring are crucial to achieve optimal control of clinical activity, and an appropriate therapeutic response. Once the therapeutic objective has been achieved, quarterly follow-up seems reasonable.
- •
Recommendation 5. The cardiovascular risk profile must be taken into account on assessment and during the therapeutic management of these patients.
- •
Recommendation 6. The most appropriate therapeutic decision will depend on the judgement of the specialist, and will be agreed with the patient. This decision will be taken based principally on the scientific evidence, and the features of the patient and their disease.
The clinical presentation of PsA is very heterogeneous and includes articular and extra-articular manifestations.1,4 As the specialists with the best knowledge and experience in the clinical and therapeutic management of this disease, rheumatologists should be in charge of diagnosis and treatment.9,13,14 However, due to the diversity of its clinical expression and associate comorbidities, multidisciplinary management of these patients is important.15–17
The clinical objective when treating patients with PsA is not as well defined as that of RA.18–22 Irrespective of the activity index used to monitor clinical activity, the priority should be to control inflammation as soon as possible, and improve the functional capacity and quality of life of patients with PsA.23–25 Clinical remission can be difficult to achieve, especially in long-term PsA.26–28 In this subgroup it can be sufficient to obtain minimal/low disease activity—to achieve 5 of the 7 criteria proposed, from musculoskeletal and skin manifestations to assessment of the patient him or herself.24,29,30 Although there is no consensus on the best tool to monitor clinical activity, the use is recommended of validated and quantifiable indices that cover parameters of inflammatory activity (joint involvement [peripheral and/or axial], dactylitis, enthesitis and acute phase reagents), and more subjective parameters that impact the patients’ function and quality of life (pain, fatigue, overall assessment of the patient, functional capacity and quality of life).22,23,31–34
A recent clinical trial has shown better outcomes after strict monitoring (tight control, every 4 weeks) compared to routine clinical practice (every 12 weeks).35 Although, at present, the best monitoring interval for patients is not clear, it seems reasonable to perform stricter control (every 4 weeks) after the disease has been diagnosed or whenever the response to a treatment needs to be assessed. When the therapeutic objective has been achieved, quarterly monitoring is sufficient.9,35
Several guidelines are currently available for the management of PsA patients that constitute essential tools for the therapeutic approach to these patients.9,36 In clinical practice, however, it is recommended that the comorbidities associated with the disease should be considered when making therapeutic decisions, as well as the patient's opinion, explaining the risk/benefit of each, because this can encourage better treatment adherence and compliance.
Approaching cardiovascular risk in patients with chronic inflammatory diseases is important, since there is a known connection between inflammation, endothelial dysfunction and increased atherogenesis.37 An increase in cardiovascular risk factors (hypertension, diabetes, dyslipidaemia, etc.) has been observed in patients with PsA associated with the role of inflammation, resulting in a greater prevalence of cardiovascular events.38 Obesity prevention is another significant aspect that we need to stress, since as well as being a cardiovascular risk factor it can also be associated with a poorer response to immunosuppressive treatments, and greater difficulty in achieving a status of minimal disease activity.39
We infer from a systematic review of this field, that the epidemiological data are insufficient to reach definitive conclusions on the effects of biological drugs and cDMARDs on cardiovascular events in patients with PsA. However TNFi and methotrexate (MTX), acting as inflammation inhibitors, can have cardioprotective effects.40
Furthermore, the specialist's therapeutic decision should be the most cost-effective possible with the greatest clinical benefit for the patient, without constituting an added burden for the national health system.
Specific RecommendationsEarly Intervention- •
Recommendation 7. Early intervention with cDMARDs is recommended for patients with PsA, principally those with poor baseline prognostic factors, in order to improve the signs and symptoms, functional capacity, and quality of life (LE: 4, GR: D).
Although the scientific evidence is sparse and controversial in this field, six studies were found that answered this question indirectly. Long duration of the disease,41,42 and delayed diagnosis of early PsA43 are major factors influencing progression of structural joint damage.
Various studies have assessed functional capacity in early and established PsA.35,43,44 It has been observed that delayed diagnosis, smoking, advanced age, being female, and a history of TNF-I treatments are associated with poorer functional capacity.41,43
It was found in the Swedish Early PsA Registry that a shorter duration of symptoms, a low baseline HAQ, and axial disease in males were independent predictive factors of minimal disease activity.44 The authors suggest that early diagnosis in patients with polyarticular involvement can be important to start specific treatment more promptly.44 Therefore, although there are few studies that cover this subject, we can consider the polyarticular forms with raised inflammatory reactants, delayed diagnosis and therapy, dactylitis, the presence of baseline erosions, and smoking to be factors of poor prognosis.
The TICOPA study demonstrated that early therapeutic intervention, and tight clinical follow-up (every 4 weeks) achieved better clinical responses (assessed by ACR and MDA) than the routine clinical practice (every 12 weeks) in a cohort of early PsA, although they found no differences in radiographic progression at 48 weeks,35 largely due to the poor overall progression of both cohorts. On the other hand, an open study performed on 35 patients with PsA observed that a delay of 3 months in starting treatment with MTX had no relevant clinical impact.45
Peripheral ArthritisNonsteroidal anti-inflammatory drugs (NSAIDs) and oral corticosteroids, used at the minimum necessary dose for the shortest possible time, can be useful as symptomatic treatment for peripheral PsA, without causing a delay in initiating disease-modifying treatment for patients for whom it is indicated. Local therapy in the form of corticosteroid injections is particularly recommended for enthesitis and dactylitis, and is also useful for peripheral mono- or oligoarticular arthritis. Ultrasound guidance can be useful for these procedures.
Synthetic Disease-modifying Anti-rheumatic Drugs (DMARDs)Conventional Synthetic Disease-modifying Anti-rheumatic Drugs (cDMARDs)- •
Recommendation 8. The cDMARDs (MTX, leflunomide, sulfasalazine) are recommended as first-line treatment for active peripheral PsA (LE: 2b, GR: C).
- •
Recommendation 9. MTX is recommended as first choice, due to its effects on arthritis and psoriasis (LE: 4, GR: D).
The cDMARDs are recommended as the first therapeutic option for patients with peripheral PsA. MTX is the treatment of choice and leflunomide and sulfasalazine are other valid options. Clinical trials of MTX, due to different methodological circumstances, have not provided conclusive data on its efficacy for PsA.46 However, extensive experience in routine clinical practice, and data from observational studies and registries suggest that MTX is effective for PsA. In the Norwegian registry, survival for MTX at 2 years was 65%,47 and in the TICOPA35 study, 22% of the patients on MTX as monotherapy achieved minimal disease activity. As yet, there is insufficient evidence for the role played by the cDMARDs in inhibiting structural damage, although MTX at high doses might have some effect.49 For all these reasons, MTX is recommended as the first therapeutic option based on broad clinical experience, its efficacy in areas such as the skin,50 its low cost and universal access.
Targeted Synthetic Disease-modifying Antirheumatic Drugs (tDMARDS)- •
Recommendation 10. Apremilast is recommended for the treatment of peripheral arthritis, after cDMARD failure or intolerance, when it is considered more appropriate than biological therapy due to the patient's profile (LE: 2b, GR: C).
Apremilast is a small molecule that inhibits phosphodiesterase 4 (PDE 4). PDE4 inhibition causes an increase in intracellular levels of cyclic adenosine monophosphate (cAMP), modulating inflammatory cytokine expression.51 The efficacy data on apremilast for peripheral arthritis, based on the results of clinical trials, seem to be inferior to biological therapy.52–54 The lack of data on radiographic progression, lack of experience in its use, and the lack of comparative studies with cDMARDs or biological agents, are currently giving rise to some doubt as to its place in the peripheral PsA therapeutic algorithm. However, its good safety profile supports is use in patients for whom other therapeutic options are not suitable, due to comorbidities or a history of severe infections.55 In addition, it can promote weight loss (between 5%-10%), which is of interest for PsA patients who are overweight or obese.
Biological Therapies (TNFi, Anti-IL12/23, Anti-IL17)- •
Recommendation 11. Biological therapy is recommended for peripheral PsA patients refractory to at least one cDMARDs (LE: 1b, GR: A).
Biological therapy would be indicated for patients with peripheral PsA where DMARDs have proved ineffective, or been discontinued due to intolerance. Various clinical trials have demonstrated that the TNFi agents are effective in all the domains of PsA. They have also been demonstrated to have a significant effect on structural damage inhibition.48,56–58 We also now have 2 new agents with a different mechanism of action, ustekinumab (i-IL12/23),59–61 and secukinumab (i-IL17),62,63 which have been recently shown to be effective in controlling the manifestations of PsA and in radiographic damage inhibition. Therefore, they are equally valid options for PsA patients and when there has been an inadequate response to DMARDs, especially for cases with severe skin involvement.
Despite the lack of clinical trials that directly compare the diverse molecules available, the differences between them do not appear significant. They are all good treatment options in the event of synthetic DMARD failure. However, based on years of experience in clinical practice and that reflected in the different international registries, the panel of experts suggest the TNF inhibitors as the first option. The other drugs are equally valid options, however, and therefore the physician's judgement must prevail.
Table 3 shows the biological therapies with their current indication for the treatment of PsA in our country.
- •
Recommendation 12. Biological therapy is recommended, in monotherapy and in combination with cDMARDs, for all the peripheral manifestations of PsA. Combining the therapy with MTX can increase the survival of the monoclonal, especially the chimeric, TNFi drugs (LE: 2b, GR: C).
Biological Therapies Available for the Treatment of Psoriatic Arthritis, According to the Datasheet.
| Biological therapy | Active ingredient | Dosage and administration | Indications | Contraindications | Adverse eventsa |
|---|---|---|---|---|---|
| TNFi alpha | Adalimumab | -Dose: 40mg -Route: subcutaneous -Frequency: every 2 weeks | Active and progressive psoriatic arthritis in adults when the response to previous therapy with DMARDs has been insufficient | -Hypersensitivity to the active ingredient or excipients | -Very common: reaction at the injection site (pain, redness) |
| -Active TBC, serious infections such as sepsis and opportunistic infections | -Common: headache, respiratory, urinary infection, herpes, diarrhoea | ||||
| -Moderate to severe HF (NYHA classes III/IV) | -Not very common: SLE, TBC, arrhythmia, sepsis, cytopenia | ||||
| -Rare: CHF, multiple sclerosis, lymphoma, solid malignant tumour | |||||
| Certolizumab | -Dose: 200mg or 400mg -Route: subcutaneous -Frequency: initially (400mg at weeks 0, 2 and 4), maintenance (200mg every 2 weeks or 400mg every 4 weeks) | Active psoriatic arthritis in adults, when the previous response to treatment with DMARDs has been inadequate | - Hypersensitivity to the active ingredient or excipients | -Very common: none | |
| -Active TBC, serious infections such as sepsis and opportunistic infections | -Common: bacterial and viral infections, leukopenias, headache, AHT, nausea | ||||
| -Moderate to severe HF (NYHA classes III/IV) | -Not very common: sepsis, TBC, fungal infections, lymphatic system neoplasms, solid tumours, non-melanoma skin cancers | ||||
| -Rare: polycytopenia, splenomegaly, melanoma, pericarditis, ILD, pneumonitis | |||||
| Etanercept | -Dose: 25 or 50mg -Route: subcutaneous -Frequency: 25mg twice per week (interval of 72–96h); 50mg once a week | Active psoriatic arthritis in adults, when previous response to treatment with DMARDs has been inadequate | -Hypersensitivity to the active ingredient or excipients | -Very common: reaction at the injection site, respiratory, urinary, skin infection | |
| -Sepsis or risk of sepsis | -Common: allergy, autoantibodies | ||||
| -Active infections (including chronic or localised) | -Not very common: serious infections, thrombocytopenia, psoriasis | ||||
| -Rare: pancytopenia, TBC, SLE | |||||
| Golimumab | -Dose: 50mg -Dose: 100mg in patients with psoriatic arthritis, with a body weight of more than 100kg who have not achieved an appropriate clinical response after 3 or 4 doses, the dose of golimumab can be increased to 100mg administered once a month -Route: subcutaneous -Frequency: once a month, the same day every month | Alone, or in combination with MTX, indicated for the treatment of active and progressive psoriatic arthritis in adults, when the response to previous treatment with DMARDs has been inadequate | -Hypersensitivity to the active ingredient or excipients | -Very common: upper respiratory tract infection | |
| -Active TBC, serious infections such as sepsis and opportunistic infections | -Common: cellulitis, herpes, bronchitis, sinusitis, AHT, superficial fungal infections, anaemia, antibodies, allergic reaction, depression, insomnia, headache | ||||
| -Moderate or severe HF (NYHA classes III/IV) | -Not very common: TBC, CHF, sepsis, neoplasms, ↑ glucose, lipids, thrombosis, arrhythmia, eye conditions | ||||
| -Rare: hepatitis B reactivation, lymphoma, pancytopenia | |||||
| Infliximab | -Dose (according to body weight): 5mg/kg -Route: i.v. infusion over 2h -Frequency: after the first dose, another at 2 and at 6 weeks. Then 1 every 6–8 weeks | Active psoriatic arthritis in adults, when previous response to treatment with DMARDs has been inadequate Should be given in combination with MTX or used in monotherapy in the event of contraindication or intolerance | -Hypersensitivity to the active ingredient, excipients or other murine proteins | -Very common: injection reaction | |
| -Active TBC, serious infections such as septicaemia, abscesses and opportunistic infections | -Common: headache, respiratory infection, herpes, diarrhoea | ||||
| -Moderate to severe HF (NYHA classes III/IV) | -Not very common: SLE, TBC, sepsis, cytopenia | ||||
| -Rare: CHF, multiple sclerosis, lymphoma | |||||
| i-IL17A | Secukinumab | -Dose: 150mg -Dose refractory to previous biological therapy: 300mg -Route: subcutaneous -Frequency: initially at week 0, 1, 2 and 3. Then monthly maintenance, starting in week 4 | Active psoriatic arthritis in adult patients who have shown inadequate response to treatment with DMARDs | -Severe hypersensitivity to the active ingredient or one of its excipients | -Very common: upper respiratory tract infections |
| -Clinically relevant active infections (e.g., active TBC) | -Common: oral herpes, rhinorrhoea, diarrhoea | ||||
| -Not very common: urticaria, conjunctivitis, neutropenia, oral candidiasis, athlete's foot, otitis externa | |||||
| -Rare: anaphylactic reactions | |||||
| i-IL12/23 | Ustekinumab | -Initial dose of 45mg administered subcutaneously followed by a second dose of 45mg 4 weeks later and then every 12 weeks -As an alternative a dose of 90mg can be given to patients who weigh over 100kg | Active psoriatic arthritis in adult patients who have shown inadequate response to treatment with DMARDs | - Severe hypersensitivity to the active ingredient or one of its excipients | -Very common: nasopharyngitis and headache |
| -Clinically relevant active infections (e.g., active TBC) | -Common: myalgias, back pain, tiredness, diarrhoea, dizziness | ||||
| -Not very common: viral respiratory tract infections, mycotic infection, depression, pustular psoriasis, injection site reactions | |||||
| -Rare: anaphylactic reactions | |||||
The data in this table were obtained from the data sheet of the Spanish Agency of Medicines and Medical Devices (AEMPS).
ILD: interstitial lung disease; DMARDs: disease-modifying antirheumatic drugs; AHT: arterial hypertension; HF: heart failure; CHF: congestive heart failure; i.v.: intravenous; SLE: systemic lupus erythematosus; MTX: methotrexate; NYHA: New York Heart Association; TBC: tuberculosis; TNF: tumour necrosis factor.
Although the usefulness of biological therapy in combination with DMARDs has been demonstrated, and is specifically recommended by SER,64 there is more controversy surrounding its use for PsA. There are no direct comparisons of efficacy and safety between the combined treatment of MTX and biological therapy, and biological therapy in monotherapy for PsA. The data from clinical trials show no significant differences in terms of efficacy (ACR responses) or safety outcomes between patients on combined treatment and patients with biological treatment in monotherapy.55,57,63–76
Therefore, no valid conclusions can be drawn of efficacy or safety for each biological drug combined with MTX compared with biological therapy in monotherapy. In general, the combination with MTX showed no significant clinical improvement.65 However, in some registries, combined therapy with MTX provides greater survival of the drug, especially in monoclonal antibodies, and particularly with infliximab, therefore it could be considered for use in this circumstance.77–80
- •
Recommendation 13. In patients with peripheral PsA, and after failure of a TNFi, a change to another biological therapy is recommended, either another TNFi or a drug with a different mechanism of action, such as i-IL12/23 or i-IL17 or a tDMARD (apremilast) (LE: 1b, 2b; GR: B).
The data from clinical trials indicate that response to a second TNFi agent is good, although inferior in general to that achieved in patients with no previous exposure to these drugs. The registries show a slight reduction in the survival of the second biological agent compared to the first, and clearly poorer survival for the third.
There are no studies that compare the usefulness of a second TNFi compared to a change of therapeutic target (IL12/23 or IL17), and therefore both therapeutic options are currently equally valid. In the studies on ustekinumab and those on secukinumab, as with the TNFi drugs, it has been demonstrated that the response to these drugs in patients with no previous exposure to biological agents is better if compared to the response obtained in patients where a TNFi has already failed. Therefore, the expected efficacy will always be better the earlier we use the biological drug, irrespective of which.63,81–90 Apremilast has also shown better ACR20 responses in patients with no previous exposure to biological therapy.53
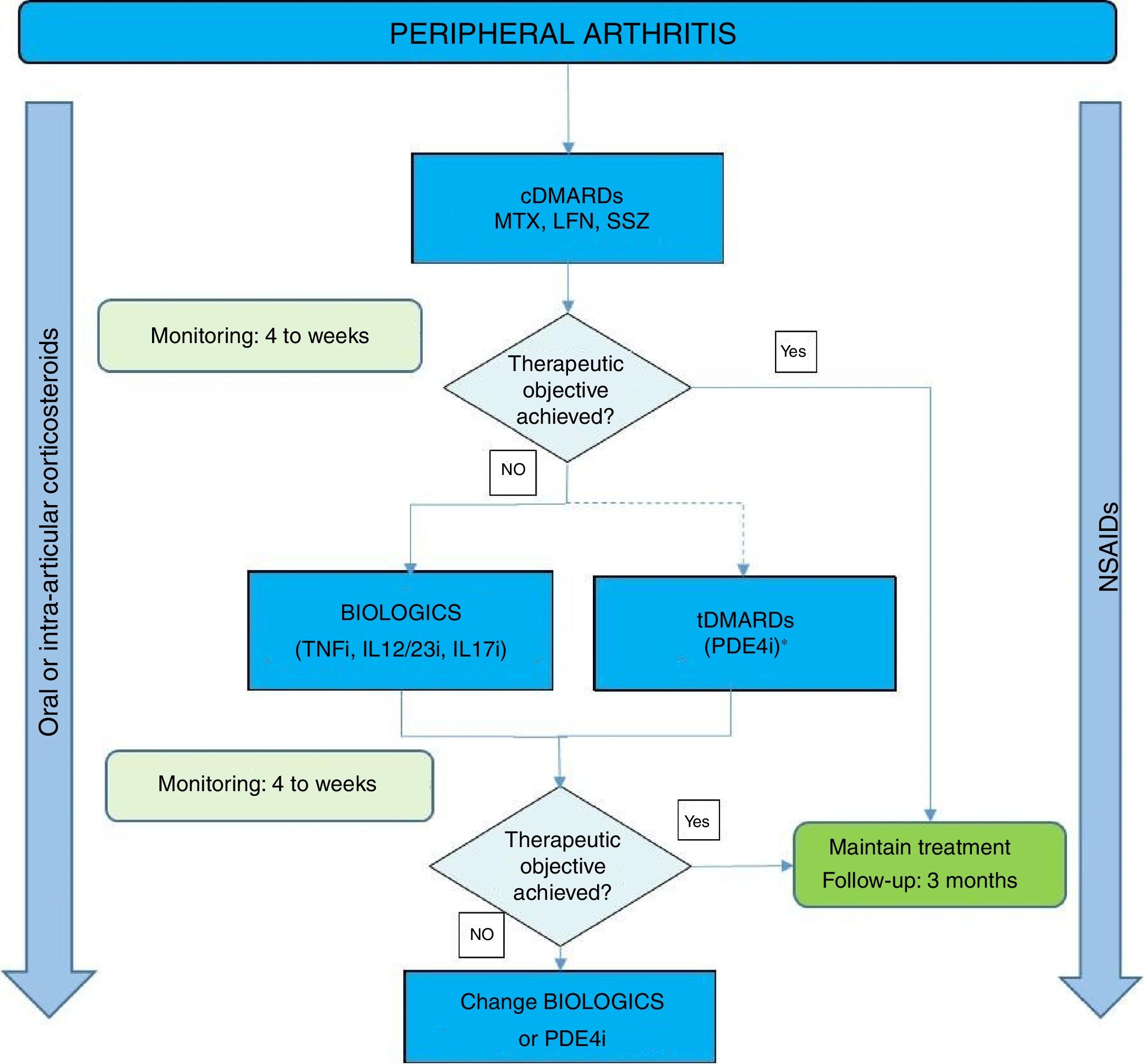
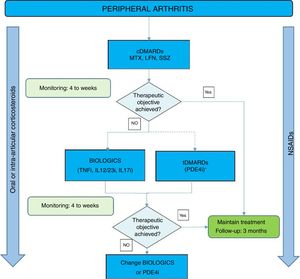
Fig. 1 shows an algorithm for the management of peripheral arthritis.
Peripheral arthritis treatment algorithm.
DMARDs: disease-modifying antirheumatic drugs; cDMARDs: conventional DMARDs; tDMARDs: targeted DMARDs; TNFi: tumour necrosis factor inhibitor; IL12i, IL23i or IL17i: interleukin inhibitor 12, 23 or 17; PDE4i: phosphodiesterase 4 inhibitor. LFN: leflunomide; MTX: methotrexate; SSZ: sulfasalazine.
- •
Recommendation 14. Biological therapy or tDMARDS (apremilast) are recommended for patients with PsA and enthesitis refractory to NSAIDs and local treatment (LE: 2b, GR: C).
A NSAID, physiotherapy and local peri-enthesis corticosteriod injections are recommended first for the forms of PsA with predominantly enthesitis involvement, although to date there are no randomised and placebo-controlled studies to vouch for their efficacy. There is no evidence to support the use of tDMARDS for enthesitis. However, tDMARDS can be considered for patients with PsA and enthesitis as long as there is associated peripheral arthritis. If a good response is not achieved despite the aforementioned treatment, the appropriate option would be biological therapy or apremilast.91 The TNFi drugs92 and subsequently ustekinumab,59,60 secukinumab,63,82 and apremilast54 have proven efficacy in the treatment of enthesitis, but there is no data to show the clear superiority of one drug over the rest. For this reason, they would all be good treatment options for patients refractory to NSAIDs and/or local treatment. However, based on years of experience in clinical practice and the international registries, the panel of experts suggest the TNFi as the first option; the other drugs are equally valid options, and therefore the final decision must be that of the physician.
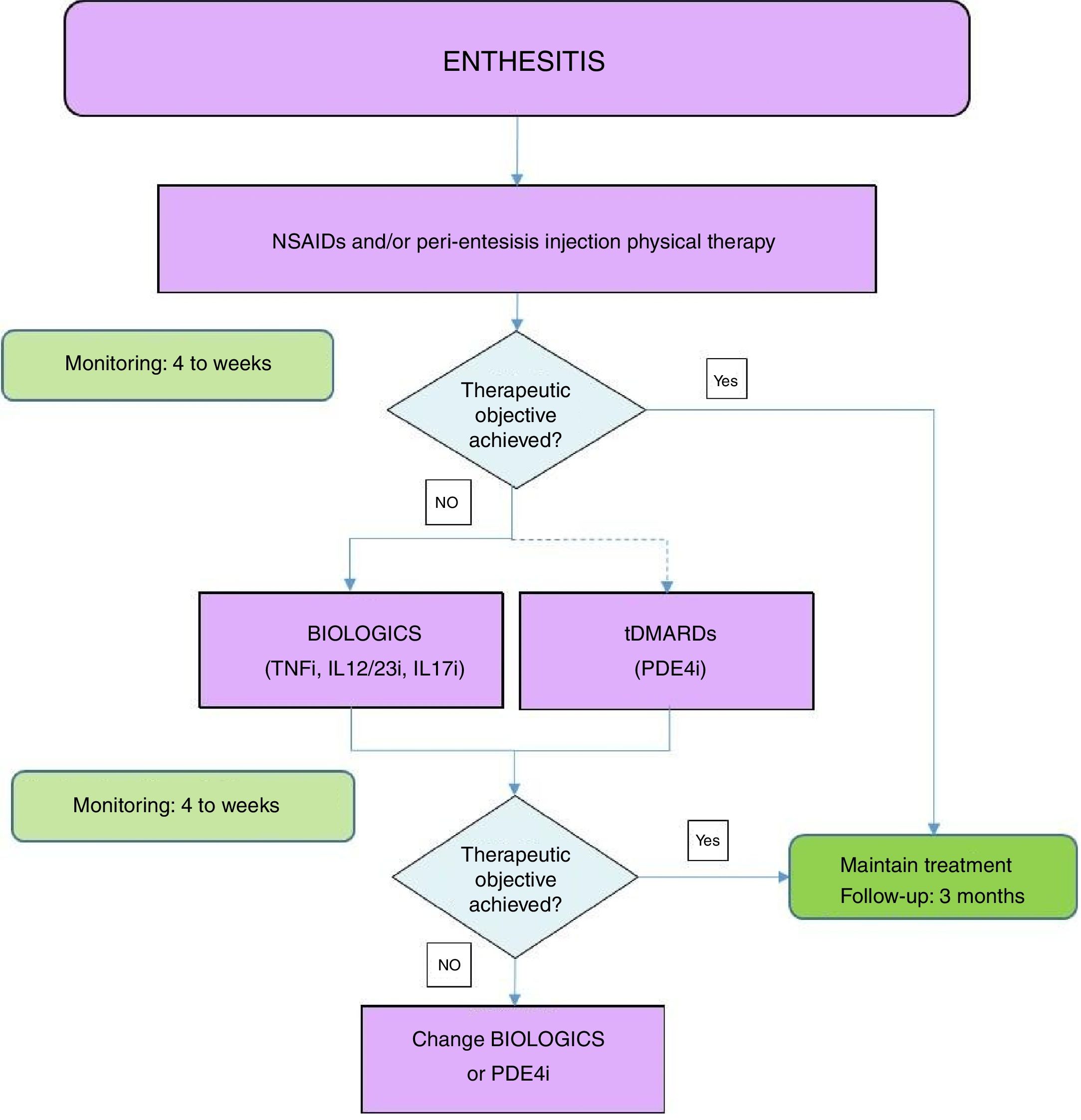
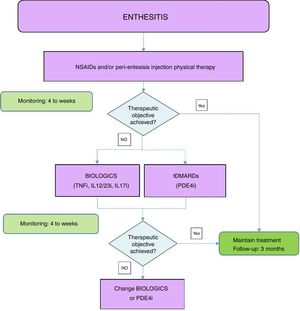
Fig. 2 shows an algorithm for the management of enthesitis.
Enthesis treatment algorithm.
NSAIDs: Non-steroidal anti-inflammatory drugs; DMARDs: disease-modifying antirheumatic drugs; tDMARDs: targeted DMARDs; TNFi: tumour necrosis factor inhibitor; IL12i,IL23i or IL17i: interleukin inhibitor 12, 23 or 17; PDE4i: phosphodiesterase inhibitor 4.
- •
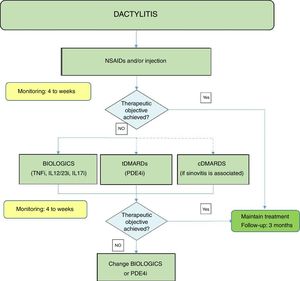
Recommendation 15. Biological treatment or tDMARDS (apremilast) are recommended for patients with PsA and dactylitis refractory to NSAIDs and local corticosteroid injections (LE: 2b, GR: C).
For the forms of PsA with dactylitis the first recommendation is NSAIDs and local corticosteroid injections, although at present we do not have randomised and placebo-controlled studies to vouch for their efficacy. The cDMARDs could be considered for patients with PsA and dactylitis as long as there is associated peripheral arthritis. The cDMARDs have an effect size that is generally small for pure dactylitis.92 The data for the TNFi drugs,92 ustekinumab,59,60 secukinumab,63,82 and apremilast54 is favourable for dactylitis: none of the molecules have been demonstrated as superior over the rest. These drugs are a therapeutic option for patients for whom local measures have failed. However, based on our years of experience in clinical practice, and the experience reflected in the different international registries, the panel of experts suggest the TNFi as the first option. The other drugs are equally valid options and therefore the judgement of the physician should prevail.
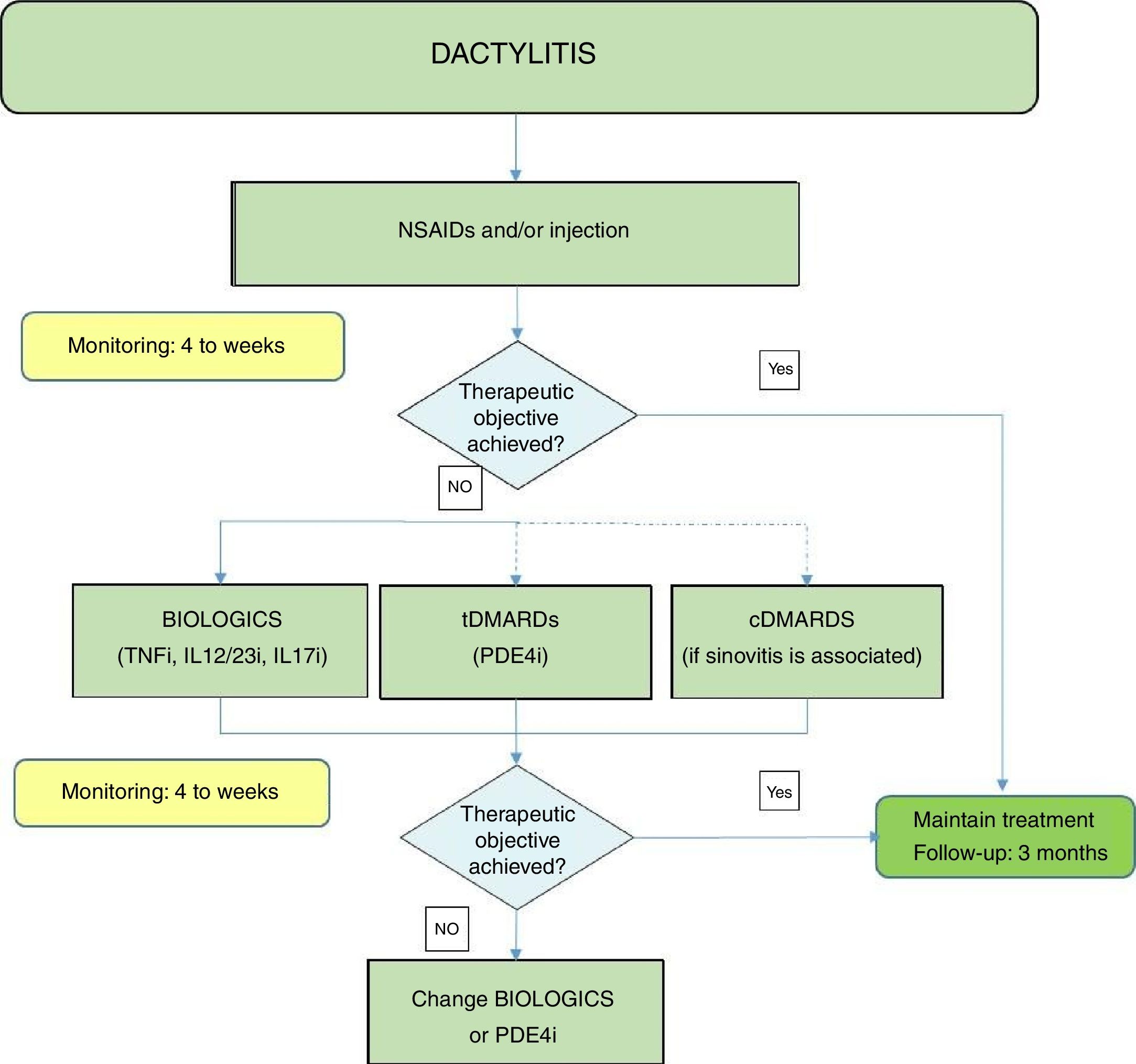
Fig. 3 shows an algorithm for the management of dactylitis.
Dactylitis treatment algorithm.
NSAIDs: Non-steroidal anti-inflammatory drugs; DMARDs: disease-modifying antirheumatic drugs; tDMARDs: targeted DMARDs; TNFi: tumour necrosis factor inhibitor; IL12i, IL23i or IL17i: interleukin inhibitor 12, 23 or 17; PDE4i: phosphodiesterase inhibitor 4.
- •
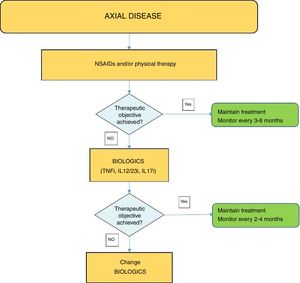
Recommendation 16. Biological therapy (TNFi or IL17i) is recommended for patients with predominantly axial forms of PsA refractory to NSAIDs, (LE: 4, GR: D).
- •
Recommendation 17. The cDMARDs are not recommended for axial forms of PsA (LE: 2b, GR: C).
In the absence of specific studies on PsA with predominant axial involvement, the general recommendations apply for axial spondyloarthritis (axial SpA), which include, in addition to physical exercise, at least 2 NSAIDs at maximum dose for a period of 4 weeks each.93 Biological treatment can be started for the patients for whom these measures prove ineffective. The use of cDMARDs is not justified because of the lack of evidence as to their efficacy at axial level. There is currently insufficient scientific evidence to indicate the use of apremilast for patients with axial SpA. A phase II, single-centre, double-blind, placebo-controlled pilot study assessed the efficacy of apremilast 30mg compared to a placebo over 12 weeks, in 36 patients with active ankylosing spondylitis.94 Although there were differences in favour of the patient treated with apremilast, they were not statistically significant, including the change in BASDAI at week 12 that was the main aim of the study.
Given the lack of comparative studies, the first biological agent should be a TNFi, in line with routine clinical practice. However, recently published data on secukinumab in ankylosing spondylitis are equally optimal,95 although at present there is no indication for this drug in the non-radiographic form.
A recent publication on ustekinumab96 of a post hoc analysis of its 2 clinical trials in PsA demonstrated a significant improvement in BASDAI and ASDAS-CRP at week 24 in the subgroup of patients with psoriatic arthritis.
At present we do not have any trials that demonstrate a significant effect of the biological therapies on structural damage in axial PsA, although there are data that show a potential slowing down effect of spinal radiographic progression associated with biological therapy on axial SpA.97,98
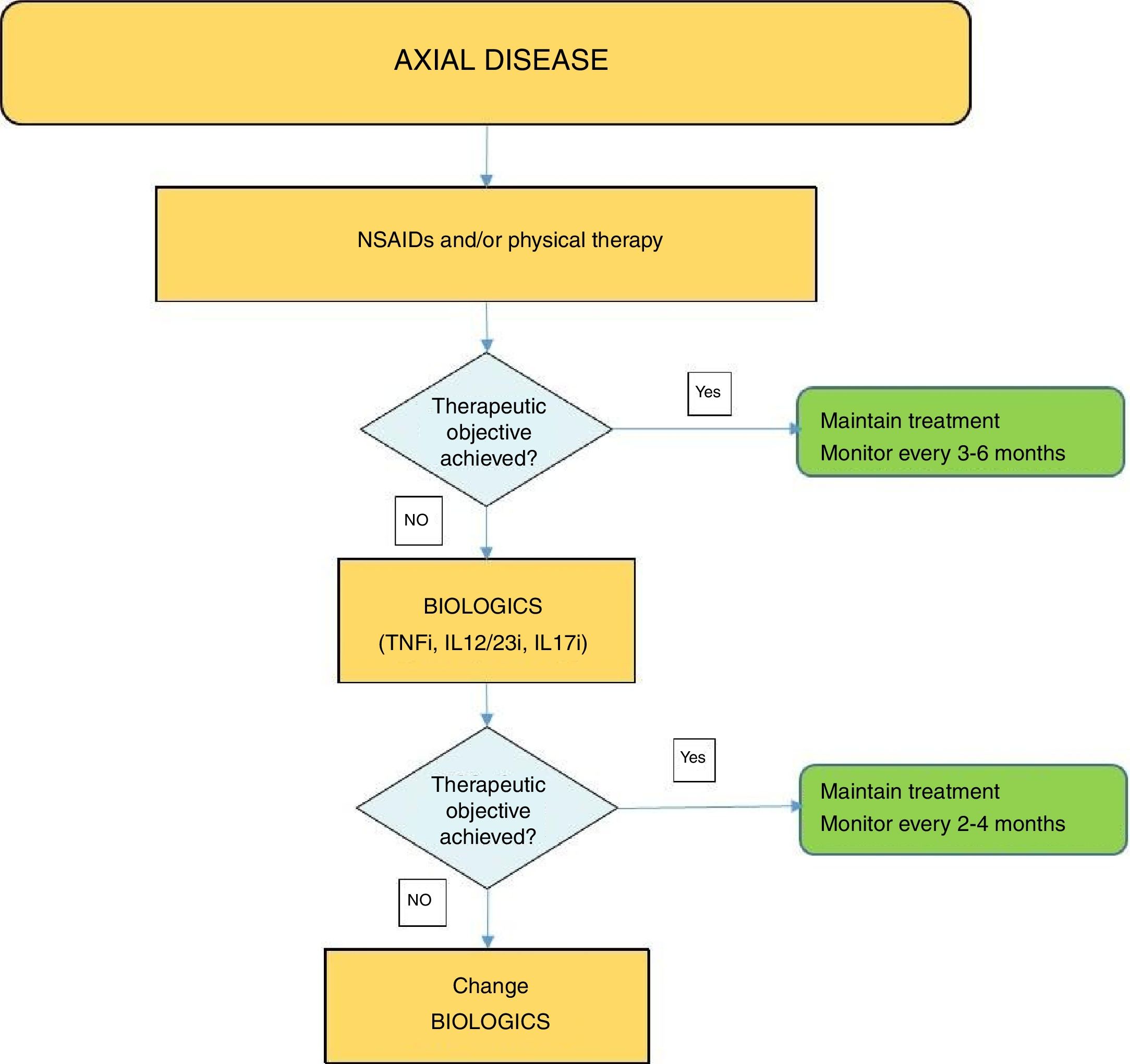
Fig. 4 shows an algorithm for the management of axial disease.
DiscussionThis new SER Recommendations document seeks to serve as a guideline for treatment using the synthetic DMARDs and biological therapy for practitioners caring for PsA patients. These recommendations, based on the best available scientific evidence and on the clinical experience of experts in PsA, are also based on the recommendations of the previous Consensus and the latest version of ESPOGUIA 2015.12
PsA is one of the most heterogeneous forms of chronic arthritis from a clinical perspective, and therefore the different therapeutic approaches and the different methods for assessing results for this entity currently pose a challenge to all clinicians in charge of diagnosing and treating this population.8 Although in recent years new drugs have been incorporated with different mechanisms of action, and different treatment modalities have been tested (T2T strategies), the overall management of PsA patients remains a challenge. Therefore, the primary aim of this recommendations document is to provide clinicians with the best available evidence (and failing this, the best opinion agreed by the panel members) for the rational and well-founded use of the various treatment options using synthetic and biological DMARDs for PsA.
The valid contributions of the previous PsA consensus document have been kept, therefore that stated in that document about cDMARDs remains in this document.11 Unlike the previous consensus document on the use of biological agents for PsA, this document provides a series of hierarchical principles for managing the disease, the best evidence has been reviewed on the new molecules that have appeared and have been approved since the previous consensus, and an algorithm is provided for the management of PsA. With all of this, the group of experts expects the management of PsA to be guided by best evidence, reducing as much as is possible the variability of this complex medical process.
The EULAR and GRAPPA recommendations for the management of PsA have been published recently.9,10 Our document, although some aspects contained in those recommendations may overlap, has been adapted as much as possible to the care reality of patients with PsA in Spain, and this could be key in generalising the recommendations contained herein.
Our document does not cover aspects relating to the skin and nail involvement of psoriasis, unlike the GRAPPA recommendations, since we consider that the management of these processes falls to the dermatologist, and the Spanish Academy of Dermatology and Venereology (AEDV) regularly publishes consensus documents similar to ours, but obviously focussing on the management of those two aspects.99
In recent years, new therapies have been added to the therapeutic arsenal against PsA due to their mechanism of action. This document has included this new evidence, especially with regard to IL12/23 and IL17 pathway inhibition (ustekinumab and secukinumab, respectively). In this respect, we decided to place the TNFi inhibitors, IL12/23-i and IL17-i, at the same level, which is also expressed in the therapeutic algorithm. This is based on the fact that, despite the absence of clinical trials that directly compare the efficacy of the diverse molecules available, the differences between them to not appear to be significant. In the section on new drugs, we have also included a new tDMARD, the PDE-4 inhibitor (apremilast). Only clinical experience will clarify the doubts as to its place in the therapeutic algorithm of PsA.7 The recommendations for these new PsA drugs are based on the best available evidence, but should be evaluated circumspectly because they are recently approved drugs. Therefore, the medium to long-term effectiveness and safety are still unknown from a real world evidence (RWE) or daily practice perspective.
PsA and psoriasis are disorders with a large group of associated comorbidities, which has led some experts to propose the concept of psoriatic disease to better capture the systemic nature of both entities.100 Cardiovascular comorbidity is perhaps the most relevant. This is why we have included specific recommendations in this regard in this document, although we recognise that this domain of the disease is in an active phase of evolution, and more evidence is needed to support the potential benefits of the synthetic and biological DMARDs in the reduction of cardiovascular risk.
The optimisation of biological therapies is an aspect highlighted in the recent literature. No specific recommendation is made in this document in this regard, since there are no consistent published data, but we do mention the need to assess this type of strategy individually. The optimisation of the biological therapies has been incorporated into the routine for managing these patients in our country, there being safety, effectiveness, cost and care equity reasons to support this type of approach. The SER and the Spanish Society of Hospital Pharmacies have recently published a positioning document for optimising biological therapy in different rheumatological diseases, including PsA.101 Even so, this aspect is principally aimed at the TNFi, since, given the very recent incorporation of other non-TNFi, it is not possible to make an extrapolation to the optimisation of these new agents.
At present, only one trial has been published on T2T approach for PsA (the TICOPA study), which seems to indicate that this management method might achieve better results in skin and joint outcomes compared to standard management, although at the cost of more adverse events.35 We believe that more information is still required on this type of approach to the disease before making a positive recommendation. Furthermore, although the evidence for early intervention with DMARDs in PsA is not supported by very solid evidence, it is obvious that early diagnosis of the disease, recognition of the adverse prognosis or poor outcome factors, and the consequent establishment of early drug intervention should set the bases for better management of the disease in all its domains, and ultimately, a general improvement in outcomes for PsA.102
These recommendations do not mention anything to do with the use of biosimilars in PsA either. A biosimilar is a biological drug that contains a version of the active substance of an already authorised original biological product (reference drug). With regard to its original product, a biosimilar must demonstrate high similarity in terms of quality, biological activity, safety and efficacy, aspects that should be contrasted by the appropriate randomised clinical trials. At present, the safe use of these products in our country is guaranteed thanks to the regulatory framework in terms of quality, preclinical and clinical criteria established by regulatory authorities such as the EMA. The interchangeability and therapeutic substitution of these drugs should not be automatic or based on purely economic criteria, since the benefit to the patient must always prevail in our decisions. Therefore, the panel of experts making these recommendations concur with what has already been expressed by the SER in their positioning document.103
In sum, this new recommendation document combines the best available evidence, incorporating the evidence on the new molecules and new PsA treatment approaches, together with the vision of rheumatologists who are experts in this disease. The essence of this manuscript lies in collecting all the aspects that help the clinician to make a reasoned therapeutic decision for a case of PsA. This means that, when choosing a particular drug, the decision should include aspects of the disease itself (clinical phenotype, severity, poor prognosis factors) together with other aspects of each molecule (evidence, experience, efficacy, safety, optimisation). In some cases, financial costs might be an aspect to be taken into account, especially when differences cannot be established based on scientific evidence, without implying an absolute limitation of medical judgement, which is the basis of the final decision.
It will soon be necessary to review and update the recommendations contained in this document, since new molecules and new treatment methods are on the short-term horizon, and we will probably have better evidence on aspects such as the therapeutic role of current and future treatments on cardiovascular risk, the use of biosimilars, and the optimisation of the current and future biological therapies.
Research AgendaAlthough these recommendations should help towards a better approach to PsA, the expert panel acknowledges that many aspects are still to be covered in a future research agenda. Some of these aspects include:
- •
Assessing from a RWE perspective the true effectiveness and safety of the new molecules approved for PsA.
- •
Studying whether there is a particular PsA phenotype that would accommodate the initial use of biological therapy without the prior need for synthetic DMARDs.
- •
An analysis of the therapeutic role of combining new synthetic DMARDs (apremilast) with biological therapy or conventional synthetic DMARDs.
- •
Assessing pharmacogenomic biomarkers of therapeutic response.
- •
An in-depth examination of the therapeutic effects of the current and future molecules on the reduction of cardiovascular risk in this population.
- •
A search for evidence to support the early therapeutic use of the current and future DMARDs on the different domains of psoriatic disease.
- •
An in-depth study of the therapeutic niche for the new molecules in the PsA treatment algorithms.
- •
Improving the optimisation strategies for the biological therapies for PsA.
- •
Extending the evidence to solidly recommend the T2T strategies.
- •
An analysis of the effect of biological therapies on the inhibition of radiographic progression in forms of axial PsA.
- •
An evaluation of the role of the biosimilars in the management of PsA.
- •
Developing pathophysiological studies on the different phenotypes of the disease to discover which cells and molecules predominate in each, in order to improve our therapeutic strategy.
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingSpanish Rheumatology Foundation.
Conflicts of InterestChamaida Plasencia Rodríguez received a grant with no restrictions from Pfizer; fees as a speaker for Pfizer.
Juan Carlos Torre Alonso received funding from MSD, Abbvie, Pfizer, Celgene, Janssen for attending courses/conferences; and has received fees as a speaker and consultant for MSD, Abbvie, Pfizer, UCB, Celgene and Novartis.
Juan D Cañete Crespillo has received funding from Novartis, Janssen and Celgene for attending meetings, conferences and courses; fees from Abbvie, Janssen and Celgene for speaking; and has received funding from Abbvie, Celgene, Boehringer, Janssen and Novartis for consultancy to pharmaceutical companies and other technologies.
Ruben Queiro Silva has received funding from Abbvie, Celgene, Pfizer, MSD, Janssen and UCB for attending meetings, conferences, courses and for speaking; funding from Janssen and Celgene for taking part in a research study; and has received financial help from Novartis, Abbvie, UCB, Celgene and Pfizer for consultancy to pharmaceutical companies and other technologies.
Mireia Moreno Martínez-Losa has received funding from Abbvie, Pfizer, MSD, Janssen, Celgene, MSD, Roche and UCB for attending meetings, conferences, courses and for speaking; and has received financial help from Janssen for consultancy to pharmaceutical companies or other technologies.
Raquel Almodóvar González has received funding from Abbvie, Pfizer, MSD, Roche for attending meetings, conferences and courses; fees from Abbvie, Celgene, Novartis, Pfizer, Roche, UCB for speaking; and has received funding from Abbvie, Jansen, MSD, Novartis, Pfizer, Roche for undertaking educational programmes and courses. And has received financial help from Abbvie, Celgene, Janssen, MSD, Pfizer, UCB for consultancy to pharmaceutical companies and other technologies.
Carlos Montilla Morales has received funding from Abbvie, MSD and Janssen for attending meetings, conferences, courses and for speaking. He has received financial help from Janssen for consultancy.
Julio Ramírez García has received funding from MSD, Abbvie, Pfizer, Bristol, UCB, Novartis, Janssen, Celgene and Roche for scientific talks and for attending meetings, conferences and training courses.
Petra Díaz del Campo Fontecha has no conflict of interests to declare.
The group of experts of this paper want to thank the rheumatology physicians who participated in the evidence review phase: Miguel Ángel Abad Hernández, José de la Mata Llord, Félix M Francisco Hernández, Jesús Maese Manzano, María Betina Nishishniya Aquino and Ana Ortiz García. They also want to thank Dr. Federico Diaz Gonzalez, Director of the SER Research Unit, for his participation in reviewing the final manuscript and for helping to preserve the independence of the document. They also want to thank Daniel Seoane, SER methodologist, and Mercedes Guerra, SER documentalist, for their collaboration in this paper.
Please cite this article as: Torre Alonso JC, Díaz del Campo Fontecha P, Almodóvar R, Cañete JD, Montilla Morales C, Moreno M, et al. Recomendaciones de la Sociedad Española de Reumatología sobre el tratamiento y uso de terapias sistémicas biológicas y no biológicas en artritis psoriásica. Reumatol Clin. 2018;14:254–268.