The aim of the systematic review was to evaluate the safety and efficacy of rituximab for the treatment of rheumatoid arthritis patients, as part of the Consensus on the use of rituximab in rheumatoid arthritis. A document with evidence based recommendations.5
MethodsAll papers published from January 2003 to September 2009 were reviewed in a systematic way in Medline, EMBASE, and Cochrane Library database. The Mesh terms used were: “Rituximab”, “Rheumatoid arthritis” “Anti-CD20”, and “Biologics”. The abstracts of the EULAR and ACR congress of 2003–2009 were also reviewed, as well as data of Roche Pharma. Two rheumatologists (BHC and MGH) made the bibliographic review by title and summary of each work. Two authors (BHC and RAA) selected them by quality according to the GRADE SCALE after review. The data was collected on paper. The outcomes evaluated were of efficacy in agreement with OMERACT13 (Outcome Measurements in Rheumatoid Arthritis Clinical Trials) and The Musculoskeletal Cochrane Study Group. The outcomes of safety evaluated were: mortality, severe infections, severe adverse events, and withdrawal for any cause, severe adverse events, and infusion related reactions. The review was conducted with Cochrane methodology. The odds ratio and relative risk for dichotomist variables, mean difference between baseline and final measurements for continuous variables, and risk differences were calculated with RevMan 5.19 The number of patients needed to treat was calculated with Cates’ calculator.20
ResultsRTX is an effective drug in 3 groups of patients with RA: patients who fail to MTX, those who fail anti-TNF and in patients with no prior exposure to MTX. It is necessary to treat 7 (5–10) patients with RTX vs placebo to obtain an ACR70 response; 9 (6–15) to achieve a DAS28 <2.6; and 5 (4–8) to achieve a HAQ improvement >0.2. The safety of the drug was similar to that of placebo except for infusion reactions where 12 (8–26) patients need to be treated with RTX vs placebo to see a reaction to the first infusion with steroid premedication. Severe adverse events to the infusion had an incidence of 0.7% in patients of the RTX treated group. It was impossible to identify a larger increase in the number of severe infections, probably due to methodological problems, however, the risk of developing infections in patients treated with RTX seems to be comparable to that of other anti-TNF and biologics.
El objetivo de la revisión sistemática fue evaluar la eficacia y la seguridad del tratamiento con RTX en pacientes con AR para la elaboración del Documento de consenso de uso de rituximab en artritis reumatoide, un documento con recomendaciones basadas en la evidencia5 sobre el empleo del fármaco en situaciones clínicas difíciles en práctica clínica habitual.
MetodologíaSe realizaron búsquedas de los trabajos publicados desde enero de 2003 hasta septiembre de 2009 en Medline, EMBASE y la Cochrane Library y revisión manual de los resúmenes de los congresos de EULAR y ACR de 2003 a 2009 y de datos proporcionados por Roche Pharma. En la estrategia de búsqueda se emplearon los siguientes términos: «Rituximab», «Rheumatoid arthritis», «Anti-CD20», «Biologics». Dos autores (BHC y MGH) efectuaron la búsqueda bibliográfica por título y resumen. Después dos autores (BHC y RAA) calificaron los trabajos según la escala GRADE y los seleccionaron tras su revisión en extenso. La extracción de los datos para el análisis se realizó en formato en papel. Las medidas de desenlace evaluadas fueron para eficacia las propuestas por OMERACT13 (Outcome Measurements in Rheumatoid Arthritis Clinical Trials) y el grupo Cochrane de Estudio de Enfermedades Musculoesqueléticas, relevantes en práctica clínica. Para seguridad se evaluaron: mortalidad, presencia de infecciones graves, efectos adversos graves, retiradas del estudio por cualquier causa, retiradas del estudio por efectos adversos graves, retiradas del estudio por reacciones a la infusión y reacciones graves relacionadas con la infusión. El análisis estadístico se realizó con el cálculo del riesgo relativo y la odds ratio para variables dicotómicas (OR) y de la diferencia media entre el valor basal frente al final para variables continuas, se estimó la diferencia absoluta de riesgo con el programa RevMan 519 y el número de pacientes necesario que tratar con las fórmulas y la calculadora de Cates20.
ResultadosEl RTX es un medicamento eficaz en el tratamiento de tres grupos de pacientes con artritis reumatoide: en fallo a MTX, fallo a anti-TNF y en pacientes sin exposición previa a MTX. Es necesario tratar a 7 (5-10) pacientes con RTX frente a placebo para obtener una respuesta ACR70; 9 (6-15) para conseguir un DAS28<2,6 y 5 (4-8) para una mejoría en el HAQ>0,2. La seguridad del fármaco fue similar a la del placebo, excepto para reacciones a la primera infusión en donde se necesita tratar 12 (8-26) pacientes con RTX frente a placebo para observar alguna reacción a la primera infusión con premedicación con corticoides. Las reacciones graves a la infusión tuvieron una incidencia de 0,7% en los pacientes del grupo tratado con RTX. No fue posible identificar un mayor incremento en el número de infecciones graves posiblemente debido a problemas metodológicos, no obstante el riesgo de desarrollar infecciones graves en pacientes tratados con RTX parece ser comparable al de otros anti-TNF y biológicos.
Rituximab (RTX) was approved in Spain for the treatment of lymphoma in June 1998 and rheumatoid arthritis (RA) in June 2006.1 Research in RA was initially based on evidence of efficacy and safety of the drug in patients with lymphoma, which has a particular clinical development. The first detailed clinical data on the use of RTX in RA was published in 20012 and included: patients with no prior exposure to methotrexate (MTX), patients that failed MTX and/or other disease-modifying drugs (DMARDs), and patients failing treatment with drugs that block tumor necrosis factor alpha (anti-TNF-α). Currently, there are more than 7 years of experience with the use of RTX in patients with RA3 and Spain has an increasingly larger number of patients with RTX, not only with RA but patients with severe and refractory manifestations of lupus erythematosus, vasculitis, Sjogren's syndrome, and other indications, showing good results. A recent meta-analysis showed that the number of patients needed to treat (NNT) with RTX vs placebo for ACR50 improvement was 4.1 (2.02–8.33).4 While the number of patients needed to harm (NNH) and see an adverse event, as well as non-compliance was not significant: 1.34 (0.65–2.76). This data is similar to that of other biologicals such as anti-TNF.4 As part of the “Rituximab Consensus. Evidence based recommendation”5 we decided to conduct a systematic review6 following Cochrane methodology. The aim of the review was to assess the efficacy and safety of treatment with RTX in RA patients, using clinical practice outcome measures and with the intention to assist the physician when making treatment decisions in difficult cases during everyday patient consultation.
Patients and MethodsSelection of studies: We searched for publications during the period of January 1, 2003 to September 30, 2009 in the following electronic databases: Medline through Pubmed,7 and the Cochrane EMBASE8 Library.9 We also performed a manual search of EULAR and ACR meeting abstracts from 2003 to 2009, and Roche Pharma10 data, as well as reviewing the Mabthera 20091 Summary. The search strategy was similar for all bases: MESH terms and free text for the following terms: rituximab, rheumatoid arthritis, anti-CD20, and biologics; we did not select any publication language filter or type of study. In a first stage, 2 authors (BHC and MGH) searched the literature independently and reviewed the title and abstract. In a second step 2 reviewers independently (BHC and RAA) selected papers after an at-length review. The final selection of items was made after consensus for any lack of agreement, considering the following criteria:
- -
Inclusion of patients over 18 years of age.
- -
RA according to 1987 criteria of the American College of Rheumatology.11
- -
Randomized clinical trials of RTX vs placebo.
We removed subanalysis studies, observational studies and duplicate studies, in the latter choosing the most recent study.
Once articles were selected, data was extracted for analysis and carried out in paper format and independently by 2 reviewers (BHC and RAA) with the Cochrane6 Collaboration tool for data extraction, which includes information on the type of clinical trial, patient characteristics, number of centers, type of intervention, efficacy and safety outcomes, and features analysis. Finally, selected studies were those whose quality score, assigned by each reviewer and according to the GRADE scale and independently graded was good or very good.12
The estimate of the possibility of bias was performed using the Cochrane collaboration6 tool, which includes assessment of: (1) identification of randomization sequence generation; (2) quality of randomization masking; (3) masking the patient and assessors; (4) similarity of the treatment groups; (5) differences between groups in follow-up; (6) differences reported in outcomes between groups; (7) differences in the presence of adverse events in the groups; and (8) evaluation of the study quality.
Outcome measures: Proposals were selected by OMERACT13 (Outcome Measurements in Rheumatoid Arthritis Clinical Trials) and the Cochrane musculoskeletal diseases study, which are detailed below:
- -
Clinical efficacy:
- 1.
Remission considered as DAS28 (Disease Activity Score), DAS28≤2.6.14
- 2.
Patients with good clinical response according to EULAR criteria (European League Against Rheumatism), those with change >1.2 and final DAS28≤3.2.15
- 3.
ACR (American College of Rheumatology) 50% (ACR50) and 70% (ACR70) criteria.16
- 4.
Physical function as measured by the HAQ (Health Assessment Questionnaire), considering an improvement in HAQ≥0.2units.17
- 1.
- -
Radiographic outcome:
- 1.
Percentage of patients without radiographic progression defined as a change in the Genant–Sharp index≤0.
- 2.
Mean change in Genant–Sharp18 radiographic index.
- 1.
- -
Safety:
- 1.
Death.
- 2.
Serious infections (infections that required patient admission to the hospital for treatment and/or endangered the life of the patient).
- 3.
Serious adverse events (those requiring hospitalization, resulting in patient death, threatened the life of the patient or left organ damage).
- 4.
Withdrawals from the study for any reason.
- 5.
Withdrawals from the study for serious adverse effects.
- 6.
Withdrawals from the study because of infusion reactions (any reaction that occurred within 24h after the infusion).
- 7.
Severe reactions associated with infusion (those that endangered the patient's life, lead to patient death, forced hospitalization or prolongation of hospitalization).
- 1.
Fort he outcomes considered we calculated the relative risk and odds ratio (OR) for dichotomous variables and mean difference from baseline compared to endline for continuous variables. For rare events, the calculation of the difference in risk was performed using a Mantel Haenszel, obtaining 95%confidence intervals (95%CI), with continuity correction when necessary. The analysis was performed using a fixed effects model and in the case of significant heterogeneity between the studies we also performed a random effects model with RevMan 5 software.19 Heterogeneity was considered as a I2≥50%.
The absolute risk difference was defined as the difference in risk of the RTX-treated group minus the difference in the risk of the placebo treated group. The NNT for efficacy outcomes of clinical and radiographic scores and the safety NNH for each outcome were calculated using the inverse of the absolute risk difference, with the Cates formulas and calculator.20
A priori, we considered the following subgroup analysis: (1) treatment with/without MTX, (2) duration of RA <2 years, (3) doses of RTX 2 cycles of 500mg vs 2 cycles of 1000mg, and (4) results at week 52.
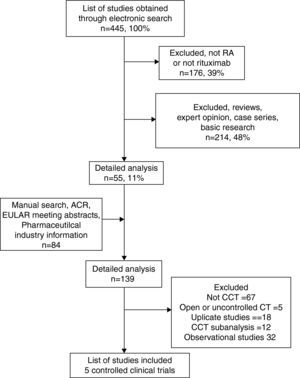
ResultsThe search identified 445 publications, of which 176 (40%) were discarded after an initial review of titles and abstracts and 234 (48%) because they were basic research studies, review papers or observational studies. We selected 55 (11%) papers for a second review, to which 84 items from 3 sources were added: a manual search, studies provided by the pharmaceutical industry, and drug data in the Archives of Pharma10 Roche. A detailed review of 139 studies was conducted and allowed the identification of 10 controlled clinical trials. Sixty-seven papers were excluded because they related to basic research, case series or were not be related to the subject; 18 were duplicate reports, 12 subanalysis or extensions of the selected clinical trials, and 32 observational studies. Finally, we selected 5 for systematic review,21–25 eliminating 5 for not having a control group or whose primary objective was not effectiveness and/or safety.26–38 The flowchart of study selection for the review is shown in Fig. 1.
The characteristics of the 5 included trials and quality assessment in accordance with GRADE12 criteria are presented in Table 1. Excluded studies and the causes are summarized in Table 2.26–38 The reasons for exclusion were methodological problems in the case of clinical trials (no placebo group, primary objective unrelated to efficacy and safety, and insufficient data to review). The papers considered included RA patients with a mean age of 51.4 years, 80% women, 84% FR +, and 8.1 years since onset of RA. All studies except the work of Tak et al.25 featured patients with failure to MTX. Two studies allowed the inclusion of patients with prior exposure to anti-TNF drugs.22,23 The patients had a high level of activity, baseline DAS28 6.7, and poor physical function (HAQ >1). Outcomes measured were clinical efficacy according to ACR, EULAR criteria, and safety outcomes in all and 2 studies included a radiographic outcome measurement.22,25 The methodological quality of the study according to the GRADE level was good, considering the quality of evidence (study design, study quality, consistency of results, and direction) and pooled within the different outcomes.
Evidence Table. Characteristics of the Studies Included in the Meta-analysis.
| Author, Year | Type | Target Population | RA Characteristics | Outcomes | Treatment Groups | Baseline | |||||||||
| Age, Years | Female % | RF+% | RA Duration, Years | DAS28ESR | HAQ | ESR mmHra | CRP mg/dl | X-ray Genant–Sharp | Quality of evidence (DRADE)* | ||||||
| Edwards et al.21, 2004n=161 | CCT | RA, MTX fail | Active: ≥8 TJC, ≥8 SJC and /or ESR≥28mmHr or CRP≥1.5mg/dl,RF+ | ACREULARSafety | Placebo+MTXRTX 2×1000RTX 2×1000+CFMRTX 2×1000+MTX | 53.7±10.7 | 78 | 100 | 10.5±6.5 | 6.85±0.8 | ND | 51 | 3.1 | ND | Good |
| Cohen et al.22, 2006n=517 | CCT | RA Anti-TNF fail1=60%, 2=31%, 3=9% | Active: ≥8 TJC, ≥8 SJC and/or ESR≥28mmHr or CRP ≥1,5mg/dlAt least one erosion | ACREULAR, X-ray,Safety | Placebo+MTXRTX 2×1000+MTX | 52.5±12.4 | 81 | 79 | 11.9±8 | 6.85±1.0 | 1.9±0.5 | 48 | 3.7 | 48.1±35.4 | Good |
| Emery et al.23, 2006n=465 | CCT | RA activeFailure to a 2–5 DMARD(≈30% anti-TNF) | Active: ≥8 TJC, ≥8 SJC, and/or ESR ≥28 mmHra o CRP ≥1,5mg/dl | ACREULARSafety | Placebo+MTXRTX 2×500+MTXRTX 2×1000+MTX | 51.2 | 80 | 82 | 10.4 | 6.75 | 1.75 | 45 | 3.1 | ND | Good |
| Emery et al.24, 2010n=512 | CCT | RA active, MTX fail, no previous biologic | Active: ≥8 TJC, ≥8 SJC and /or ESR ≥28 mmHr or CRP ≥0,6mg/dl | EULARACRSafety | Placebo+MTXRTX 2×500+MTXRTX 2×1000+MTX | 51.7±12.6 | 82 | 74 | 7.06±7.2 | 6.4±1.0 | ND | ND | ND | ND | Good |
| Tak et al.25, 2009n=755 | CCT | RA active, >4 years, No prior MTX | Active: ≥8 TJC, ≥8 SJC and CRP ≥1,0mg/dl | X-rayACREULARSafety | Placebo+MTXRTX+MTX 2×500RTX+MTX 2×1000 | 47.9±13.1 | 81 | 86 | 0.9±1.1 | 7.1±1.0 | 1.8±0.6 | ND | 3.2 | 7.6±11.0 | Good |
RA: rheumatoid arthritis; ACR: ACR response; DAS28ESR: disease activity score calculated using ESR; CCT: controlled clinical trial; EULAR: EULAR criteria for improvement using DAS28; DMARD: disease modifying anti-rheumatic drugs; FR+: positive rheumatoid factor; MTX: methotrexate; TJC: tender joint count out of 68; SJC: swollen joint count out of 66; ND: no data; CRP: C reactive protein mg/dl; RTX: rituximab; ESR: erythrocyte sedimentation rate, mm/h; 2×1000: 2 cycles of 1000mg each, on days 0 and 15; 2×500: 2 cycles 500mg each on days 0 and 15.
aQuality evaluation using Cochrane methodology.
Articles Excluded From the Meta-Analysis and the Motive for Exclusion.
| Author, Year | Type | Target Population | Outcome | Motive for Exclusion |
| Rubber Roth et al.,26 2010 | CT | Active RA, MTX fail | ACR response, EULAR response | No placebo group |
| Bingham III et al.,27 2009 | CT | Active RA | Response to vaccine | Open CT, objective other than efficacy |
| Loveless et al.,28 2009 | CT | Active RA, ≥1 DMARD fail | Safety and efficacy | Open trial, no placebo group |
| Genwald et al.,29 2009 | CT | Active RA in spite of MTX+etanercept or MTX+adalimumab | Safety (infections)efficacy | Patients treated with RTX in combination with anti-TNF |
| Mease et al.,30 2008 | CT | RA+≥1 anti-TNF fail | Efficacy and safety of a 2nd round of RTX vs placebo | No placebo group |
| Cohen et al.,31 2009 | CT22 | Active RA, anti-TNF fail | ACR, EULAR, X-ray, safety | Extension |
| Issacs et al.,32 2009 | CT22,23 | In FR+ and/or CCP+ patients | Subanalysis | Subanalysis |
| Keystone et al.,33 2009 | CT22,23 | 1000mg×2+MTX | Subanalysis, open phase, patients with initial response | Subanalysis |
| Keystone et al.,34 2007 | CT22,24 | 1000mg×2+MTX | Subanalysis of open phase, patients with initial response | Subanalysis |
| Mariette et al.,35 2009 | CT25 | 500×2+MTX1000×2+MTXPlacebo+MTX | Subanalysis RF+and/or CCP+and poor prognosis | Subanalysis |
| Emery et al.,36 2009 | CT23 vs 22,24 | 1000×2+MTX | Phase II and III subanalysis | Subanalysis |
| Kremer et al.,37 2006 | CT23 | RTX 1000×2+MTX vs Placebo+MTX | Subanalysis of REFLEX | Subanalysis |
| Vollenhoven et al.,38 2009 | CT22–27 | At least 1 cycle of RTX | Safety | Subanalysis |
The primary efficacy results at 24 weeks are summarized in Table 3. The efficacy outcomes are presented for all patients and 2 subtypes of patients with RA included in the articles: DMARD failure patients and patients with failure to anti-TNF. Analyzing all patients, an ACR50 response was found in 40% of patients treated with RTX vs 19% in the placebo group, the absolute risk difference was 21% and NNT with RTX vs placebo for an ACR50 response was 5 (4–6). There was an ACR70 response in 24% of patients treated with RTX vs 10% in the placebo group, the absolute risk difference was 14% and NNT with RTX vs placebo for an ACR70 response was 7 (5–10).
Grouped Results of Treatment Efficacy of 2 Cycles of RTX vs Placebo for All Patients and for the Groups of Patients with RA and Failure to Anti-TNF.
| Outcome (Week 24) | No. of CCT | No. | Frequency in RTX GroupNo. of Events/Total (%) | Frequency in PBO GroupNo. of Events/Total (%) | Odds Ratio (95% CI) | NNT (95% CI) |
| All scenarios | ||||||
| ACR50 | 5 | 2251 | 597/1484 (40) | 144/762 (19) | 3.1 (2.5–4.0) | 5 (4–6) |
| ACR70 | 5 | 2251 | 358/1484 (24) | 78/767 (10) | 3.0 (2.3–4.0) | 7 (5–10) |
| DAS28 <2.6 | 3 | 1723 | 194/1118 (17) | 33/605 (5) | 3.5 (2.4–5.2) | 9 (6–15) |
| Good EULAR response | 4 | 1497 | 164/982 (17) | 18/515 (3) | 5.2 (3.2–8.7) | 8 (5–15) |
| HAQ improvement >0.2 | 2 | 876 | 789/1065 (74) | 302/526 (57) | 2.3 (1.7–3.1) | 5 (4–8) |
| % of patients with no X-ray progression at week 52 | 2 | 1179 | 460/761 (60) | 208/418 (50) | 1.5 (1.2–1.9) | 10 (7–22) |
| DMARD fail | ||||||
| ACR50 | 3 | 956 | 170/582 (29) | 32/294 (13) | 3.3 (2.2–5.11) | 5 (4–9) |
| ACR70 | 2 | 876 | 62/582 (11) | 15/294 (5) | 2.2 (1.2–3.9) | 19 (9–88) |
| DAS28<2.6 | 2 | 876 | 32/377 (8) | 4/172 (2) | 4.4 (1.5–12.6) | 18 (13–29) |
| HAQ improvement >0.2 | 2 | 876 | 366/582 (63) | 123/294 (42) | 2.3 (1.7–3.1) | 5 (4–8) |
| Anti-TNF fail | ||||||
| ACR50 | 1 | 499 | 80/298 (27) | 10/201 (5) | 7.01 (3.5–13.9) | 5 (3–10) |
| ACR70 | 1 | 499 | 36/298 (12) | 2/201 (0.9) | 13.6 (3.2–57.4) | 10 (3–47) |
| DAS28<2.6 | 1 | 499 | 27/298 (9) | 0/201 (0) | NE | NE |
| EULAR response | 1 | 499 | 149/298 (50) | 40/201 (20) | 4.0 (2.6–6.0) | 4 (3–6) |
NE: not evaluated. Frequency of events in control group is 0 making calculations impossible.
Considering the outcomes that are assessed in clinical practice, we found a DAS28 <2.6 at 24 weeks of treatment in 17% of patients treated with RTX vs 5% in the placebo group; the absolute risk difference was 12% and NNT with RTX vs placebo for an DAS28 <2.6 response was 9 (6–15). The frequency of patients with a good EULAR response at 24 weeks of treatment was 17% in patients treated with RTX vs 3% in the placebo group; the absolute risk difference was 14% and NNT with RTX vs placebo for a good EULAR response was 8 (5–15). The improvement in physical function, 1 of the most relevant domains of quality of life, measured as the percentage of patients with improvement in HAQ >0.2 at 24 weeks of the first cycle of treatment with RTX was 74% vs 57% in the placebo group, with an absolute risk difference of 21% and the NNT with RTX vs placebo for an improvement in HAQ>0.2 was 6 (4–8).
The radiographic outcome was assessed in 2 of the 5 papers. The percentage of patients showing no radiographic progression at week 24 was 66% in the RTX-treated group compared with 59% of the placebo group, OR 1.34 (1.04–1.71). At 52 weeks, the percentage of patients without radiographic progression was 60% in the RTX treated group vs 50% in the placebo group, the absolute risk difference was 10%, OR 1.53 (1.20–1.95) and the NNT with RTX vs placebo and not have radiographic progression was 10 (7–22). The mean difference in the Genant–Sharp Index at week 24 was 0.57 for the RTX-treated group vs 0.95 for the placebo group and at week 52 of 0.563 vs 1.05, respectively for a mean difference of −0.6 (−1.14 to −0.06). Problems of heterogeneity found in ACR50 and HAQ improvement≥0.2 with I2=59% and 56% respectively. For the rest of the outcomes of effectiveness the I2 was 13%–42%.
When we analyzed the data including only studies with DMARD failure21,23,24 the direction and magnitude of response were similar but with higher NNT and wider confidence intervals, as shown in Table 3. The heterogeneity also increased with an I2=62% for remission and DAS28 response and an I2=82% for changes in HAQ. The same happened when the analysis was performed with patients with RA and failure to anti-TNF. In this analysis, the assessment of DAS28remission was not possible, because 27 of 298 (9%) patients in the RTX-treated group and none in the placebo group (0/201) showed remission.
Safety results are summarized in Table 4. The evaluation of safety outcomes was only possible at 24 weeks due to study designs. The frequency of any infusion reaction was higher in the group treated with RTX. The NNH of RTX vs placebo in order to present a reaction to the first infusion, when patients receive premedication with corticosteroids was 12 (8–26). In the case of serious infusion reactions, they only occurred in the group treated with RTX (0.74%), making it impossible to estimate absolute risk difference, OR and NNH. No significant differences between both groups were found regarding the number of serious adverse events, mortality, severe infections and withdrawals due to serious adverse effects. A greater number of study withdrawals due to adverse events were seen in the placebo group, due to disease activity. This estimate showed the most heterogeneity (I2=93%) and in the rest with a lower I2 it was lower than 50%.
Grouped Results Regarding Safety of Treatment in a Cycle of 2 RTX Infusions vs Placebo.
| Outcome (Week 24) | No. of CCT | No. | Frequency in RTX Group (%)No. of Events/Total (%) | Frequency in PBO Group (%)No. of Events/Total (%) | Odds ratio (95% CI) | NNH (95% CI) |
| Severe adverse events | 5 | 2327 | 123/1565 (8) | 66/762 (9) | 0.94 (0.68–1.30) | NS |
| Death | 5 | 2367 | 2/1565 (0.13) | 3/80 (0.37) | 0.33 (0.08–1.37) | NS |
| Reactions to first infusiona | ||||||
| Any | 4 | 1652 | 272/1082 (25) | 92/570 (16) | 1.70 (1.31–2.22) | 12 (8–26) |
| Severe | 4 | 1652 | 18/1082 (0.74) | 0/570 (0%) | NE | NE |
| Serious infection | 4 | 1652 | 18/1082 (1.7) | 10/570 (1.8) | 0.93 (0.45–2.05) | NS |
| Study dropout, any cause | 4 | 1652 | 99/1083 (9) | 85/569 (15) | 0.6 (0.44–0.81) | 19 (13–41) |
| Dropout due to SAE | 4 | 1652 | 23/1084 (2) | 5/370 (0.9) | 2,23 (0.87–5.69) | NS |
SAE: severe adverse event; NE: not evaluated. Frequency of adverse events in control group is 0, making calculations impossible; NNH: number seeded to harm; NS: not significant.
Infusion reactions (any reaction occurring in the 24h post-infusion.
Severe reactions related to the infusion: those that put the patients life in danger, led to death, merited hospitalization or prolonged hospital stay.
Serious infection: infections requiring hospitalization of patient for treatment and/or put the patients life in danger.
Severe adverse events: those requiring hospitalization, caused patients death, put the life of the patient in danger or led to long term complications.
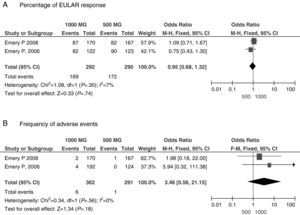
A second meta-analysis objective was to investigate the efficacy of cycles with different doses of RTX: 500mg×2 vs 1000mg×2. Patients with RA and who had anti-TNF failure received cycles of RTX at doses of 1000mg×2.22 Dose analysis included only 2 studies23,24 of patients with failure to MTX and found no significant differences in efficacy or safety variables between the 2 doses used, as shown in Fig. 2.
The administration of RTX monotherapy vs combination therapy RTX+MTX was evaluated in a single randomized trial21 including 40 patients per group; this study had the greatest risk of bias and heterogeneity and it was not possible to perform the analysis. Something similar happened when trying to assess RTX+DMARD treatment different from MTX, as the only combination found was RTX+CFM.21 A fourth sub-analysis was performed with clinical data at 52 weeks; the efficacy results were similar to those occurring at week 24 (data not shown). The safety data could be assessed only at week 24.
DiscussionThe purpose of a systematic review of therapeutic intervention is to find the best available evidence on the efficacy and safety of a drug compared to standard therapy. A meta-analysis including 5 controlled trials of adequate methodological quality was performed to insure its internal validity.
Patients included were predominantly women over 50 years of age, with established RA defined by ACR criteria, with high activity and poor physical function. Most had failed DMARD treatment (at least MTX) and/or anti-TNF, similar to those seen in clinical practice.
The findings of the meta-analysis related to efficacy outcomes have a good level of evidence. It was found that 2 cycles of RTX 1000mg each administered IV on days 0 and 15, were better than placebo in achieving a response at week 24 considering: ACR50 with NNT of 5 (4–6), ACR70 response with NNT of 7 (5–10), DAS28 remission as ≤2.6 with NNT of 9 (6–15), EULAR response with NNT of 8 (5–15), improvement in HAQ≥0.2 with NNT 5 (4–8), no radiographic progression 1.5 (1.2–1.9) and less change in the Genant–Sharp index 0.6 (−1.14 to −0.06). The methodological quality according to the GRADE scale and nature of the models used for the estimates were moderate to good; problems of heterogeneity were found for the ACR50 response and improvement in HAQ. When performing the corresponding analysis of efficacy in 2 subpopulations of patients with RA: failure to DMARD and to anti-TNF, the results were similar, but the data less robust due to an increase in model heterogeneity, with estimates of lower quality.
The quality of the results of the evaluation of the safety outcome was a major problem and was moderated by the following considerations:
- 1.
Possibility of selection bias: Trials included patients without comorbidity and exclude patients with significant comorbidity who present worse safety outcomes; also, consider that the selection process chooses high-activity patients, including patients from various geographical areas, where access to the drug may condition the entry of the patient.
- 2.
The sample size of trials was calculated for outcomes of efficacy and not safety and that is important in rare safety outcomes (mortality and severe infection).
- 3.
Methodological aspects: In the trials included in the meta-analysis, the placebo phase was 24 weeks, allowing rescue medication, which further reduced the sample size. Safety monitoring is completed when the trial ended, usually at 4 weeks after the last follow-up visit and not done in the long term, a fact particularly important when talking about a drug that can cause sustained depletion of B lymphocytes.
- 4.
Problems were found related to significant heterogeneity for withdrawals from the study for any adverse events and serious adverse events. With all these considerations, the quality of evidence for safety outcomes was moderate and this should be considered when interpreting the results. There were more infusion-related reactions of any kind (NNH 12; 8–26) and especially severe reactions related to infusion into the RTX-treated group (0.7% vs 0%, respectively), in the later case, there were no reactions in the placebo group and estimation of the NNT was impossible. The possibility of reactions during the infusion of the drug is more when undergoing the first infusion and is reduced by premedication and proper administration of the drug; serious infusion reactions will occur in approximately 0.5% of patients.1,10 There were no reports of infusion-related mortality. The methodological quality to make this assertion was moderate. For other safety outcomes: number of serious adverse effects, mortality, severe infections and withdrawal from the study due to serious adverse events, no differences were found between the RTX and the placebo group. There were a greater number of withdrawals from the trial for any cause in the placebo group vs the group treated with RTX, a low level of evidence given the heterogeneity of the estimate.
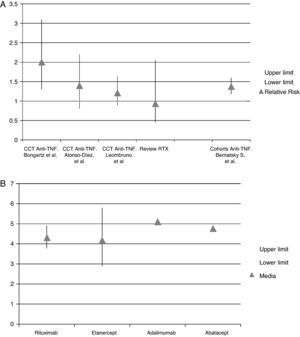
As in other previous meta-analysis, there were no significant differences in serious infections in patients treated with RTX vs placebo.39 This finding deserves a special comment: the absence of difference in serious infections between the treated and placebo groups could be a type II statistical error, i.e., assuming that no differences between groups exist when in fact there are. Similar difficulties occur in the meta-analysis of patients with RA and anti-TNF,40,41 as shown in Fig. 3A, where the results are contradictory. A recent meta-analysis of observational studies has shown an increased risk of serious infections in patients with RA receiving anti-TNF vs DMARD.42 Patients in the prospective observational studies are similar to those in routine clinical practice and could eliminate selection bias. This data is consistent with the impression of clinicians; we observed that anti-TNF drugs increase the risk of severe infections.43 The incidence of serious infections per 100 patient-years in patients treated with RTX is similar to that seen with other biologics (anti-TNF or other therapeutic targets) (Fig. 3B).31–34 Despite finding no difference in infections among patients treated with RTX vs placebo in the systematic review, we believe that the frequency of serious infections with RTX is similar to that observed with anti-TNF and other biologics.39–43
In addition to these limitations, another limitation was the timeline; and only estimates were made at week 24 for efficacy and safety variables, and in week 52 for X-ray outcomes. Other interesting questions arose when comparing efficacy at doses of 500×2 vs 1000×2, vs monotherapy. Combination therapy with DMARDs, concomitant use of different DMARDs or efficacy of MTX in patients with antibodies to citrullinated peptides and/or rheumatoid factor could not be answered given the lack of data for analysis.
The implications of these findings in clinical practice are that RTX is an effective treatment in patients with active RA, considering clinical and radiographic outcome measures. The number needed to treat was 7 (5–10) RTX patients compared with placebo for an ACR70 response, 9 (6–15) to achieve a DAS28 <2.6 and 6 (4–8) for a HAQ <0.2. The safety of the drug was similar to that of placebo, except for the first infusion reactions. The NNH for reactions to the first infusion was 12 (8–26). It was not possible to identify an increase in other safety outcomes, including the number of serious infections, possibly due to methodological problems. However, the risk of developing serious infections in patients treated with RTX seems to be comparable to other anti-TNF biologics.
Conflict of InterestThis document was financed through an unrestricted grant to the department of Rheumatology of the del Hospital Universitario La Paz (AREPAZ). The choice of reviewers was the sole responsibility of the study coordinator (EMM). No Roche Pharma employee participated in the scientific meeting or had knowledge of their development until the project was finished. The meeting organization and expert fees were the sole responsibility of the Hospital Universitario La Paz (AREPAZ).
Please cite this article as: Hernández-Cruz B, et al. Rituximab en artritis reumatoide: una revisión sistemática de eficacia y seguridad. Reumatol Clin. 2011. doi:10.1016/j.reuma.2011.03.004.