A 24-year-old woman with systemic lupus erythematosus (SLE) presented abdominal pain and diarrhea. No evidence for an SLE flare was obtained. Colonoscopy and microscopic biopsy examination revealed findings typical of Crohn's disease. Despite the rarity of the combination, patients with SLE showing gastrointestinal manifestations might merit evaluation for Crohn disease.
La asociación del lupus eritematoso sistémico (LES) y la enfermedad inflamatoria intestinal es rara. Presentamos el caso de una mujer de 24 años con LES que comenzó con dolor abdominal y diarrea. No había datos de exacerbación de LES. Las pruebas complementarias mostraron hallazgos típicos de enfermedad de Crohn.
Although many autoimmune diseases tend to coexist in patients, the association of systemic lupus erythematosus (SLE) and inflammatory bowel disease (IBD) is uncommon. Patients with SLE may be affected mainly by intestinal vasculitis and patients with IBD may have manifestations in common with SLE, such as oral ulcers, arthritis, etc. Furthermore, drugs used for IBD, such as sulfasalazine, can cause drug-induced lupus.1 We present the case of an association of both processes. In our case, the manifestations of SLE preceded IBD and there was no involvement of drugs in the manifestation of SLE.
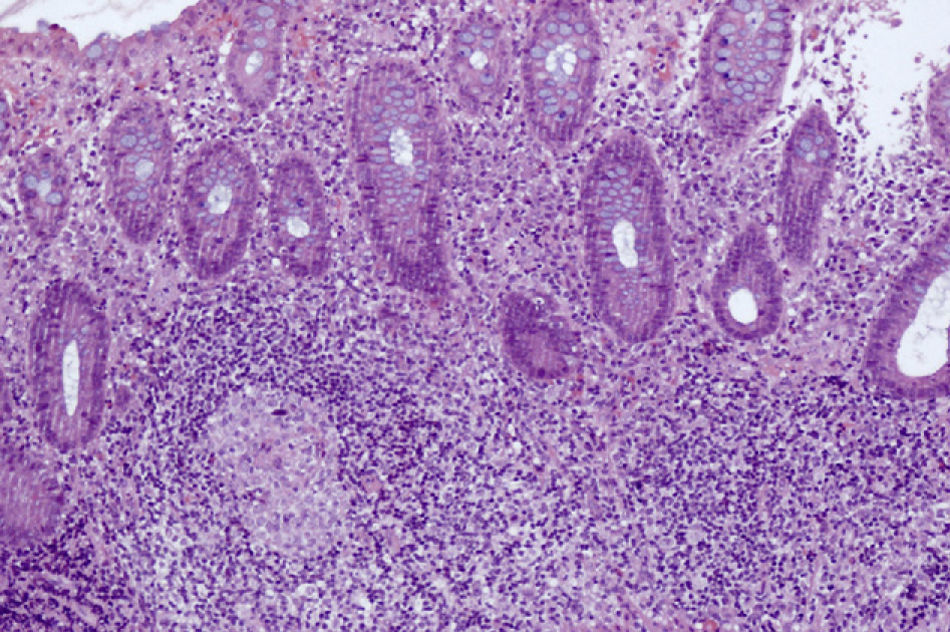
Case ReportA 24-year-old woman was diagnosed with SLE in 2002 after presenting joint pain, malar rash, polyarthritis, positive ANA 1/1.280 with a homogeneous pattern, and positive anti-dsDNA 14.2 (positive >1.1), along with histological nodal follicular lymphoid hyperplasia. She was treated with oral corticosteroids, NSAIDs, and antimalarials. In 2007, lung disease was diagnosed in relation to her underlying disease (transbronchial biopsy compatible with nonspecific interstitial pneumonitis), starting intravenous cyclophosphamide, interrupted by pregnancy, for a total of 6 standard-dose intravenous boluses (the last in May 2008). Since pregnancy she has received no immunomodulatory therapy and has presented clinical improvement. She does present anemia of chronic diseases associated with iron deficiency. No evidence of antiphospholipid antibodies in repeated determinations or pulmonary hypertension has been found. She was admitted to our hospital in March 2010 for abdominal pain and diarrhea (6–8 loose stools) lasting a week. No fever or other clinical evidence of exacerbation of SLE was found. At the time of onset of diarrhea she was under no treatment. Physical examination showed abdominal distention and tenderness in the epigastrium, hypochondrium, and right flank. The rest of the examination was normal. In laboratory tests, apart from anemia (Hb: 9.9g/dl, MCV: 68.3, MCH: 20), no other blood disorders were seen. Coagulation and hepatic and renal function tests were normal. Urinalysis showed no abnormalities. Stool culture and study of fecal parasites were negative. Serum levels of C3, C4, IgG, IgM were normal. She had positive ANA title 1/640 with a nucleolar pattern, but ENA, anti-DNA, and antiphospholipid antibodies were negative. The abdominal CT showed involvement of the segmental terminal ileum (diffuse thickening of the wall and slight decrease in size with finger-like edema) adjacent to which we found a collection 28×23mm in size, probably related with an abscess. Colonoscopy of the cecum was performed, on the right colon, cecum, and iliocecal valve, showing several moderately deep ulcers with geographic edges about 1cm in diameter, with normal mucosa between them. These findings were compatible with Crohn's disease. The colon biopsy confirmed the endoscopic findings. Microscopic examination revealed fragments of large bowel mucosa with patchy involvement at the expense of small architectural distortion, mucin depletion, and a transmural inflammatory infiltrate consisting of lymphocytes, plasma cells, neutrophils, and eosinophils with occasional granulomas and a single cryptic abscess. It was accompanied by areas of ulceration, granulation tissue, and fibrino-leukocyte material. Lymphoid accumulations were not seen (Fig. 1).
Once the diagnosis was made, treatment was started with prednisone (0.5mg/kg) and azathioprine (2mg/kg), having to suspend the latter a month later because of acute pancreatitis. She was subsequently treated with mesalazine (1000mg/8h) with rapid improvement of symptoms. At present, after 6 months of treatment, the patient is symptom free.
DiscussionSLE can affect the entire gastrointestinal tract.2,3 However, the development of gastrointestinal complications in patients with SLE is not related to drugs and infections are rare.4 Although lupus enteritis (gastrointestinal vasculitis) may be difficult to distinguish, the onset of an inflammatory intestinal disease5 in our patient with diarrhea and abdominal pain was not accompanied by a relapse of SLE. In addition, the segmental involvement on the CT and endoscopy was suggestive of Crohn's disease and the colon biopsy lacking evidence of vasculitis, as well as the above findings confirmed the result. In these cases the histological study is necessary to make the differential diagnosis between these two conditions. To establish the diagnosis of lupus enteritis requires evidence of deposits of immunoglobulins and complement in capillary walls and deposits in electron microscopy.6 As in other cases,5 we decided to treat the patient with azathioprine, which is useful in the manifestations of SLE and IBD both, but withdrew it because of toxic pancreatitis and opted for the administration of mesalazine, with good clinical response. Both SLE and IBD are chronic autoimmune diseases characterized by episodes of relapse and remission.1 The association is rare, the estimated prevalence of ulcerative colitis in patients with SLE is around 0.4% and Crohn's disease is even less.7,8 In most cases, as in ours, the diagnosis of SLE occurs prior to IBD. The first disease is usually inactive at the time the second manifests. Patients with both processes tend to have less photosensitivity, serositis, and neurological disorders, and in general have a relatively favorable prognosis of both SLE and IBD.1 Thus, despite this uncommon association, it should be taken into account. If a patient is diagnosed with SLE and begins with gastrointestinal symptoms including abdominal pain and diarrhea, especially if it is not associated with clinical symptoms of recurrence of SLE, it is prudent to rule out IBD.
DisclosuresThe authors have nothing to disclose.
Please cite this article as: Fernández Rodríguez AM, et al. Lupus eritematoso sistémico y enfermedad de Crohn: un caso. Reumatol Clin. 2012;8:141–2.