Systemic sclerosis is a connective tissue disease characterized by inflammation and fibrosis of multiple organs (skin, gastrointestinal tract, lung, kidney and heart). After the skin, the organ most affected with a frequency of 75%–90%, the gastrointestinal tract is more often involved.
Gastrointestinal tract involvement is manifested by the appearance of oropharyngeal dysphagia, esophageal dysphagia, gastroesophageal reflux, gastroparesis, pseudo-obstruction, bacterial overgrowth and intestinal malabsorption, constipation, diarrhea and/or fecal incontinence. These effects influence food intake and intestinal absorption leading to the gradual emergence of nutritional deficiencies. About 30% of patients with systemic sclerosis are at risk of malnutrition. In 5%–10%, gastrointestinal disorders are the leading cause of death.
Therapeutic strategies currently available are limited and aimed at reducing clinical symptoms. The multidisciplinary management of these patients, including nutritional intervention, helps improve gastrointestinal symptoms, and avoid malnutrition, morbidity and improve quality of life.
La esclerosis sistémica es una enfermedad del tejido conectivo caracterizada por inflamación y fibrosis de múltiples órganos (piel, aparato digestivo, pulmón, riñón y corazón). Después de la piel, el órgano más afectado, con una frecuencia del 75 al 90%, es el tracto gastrointestinal.
La afectación del tracto gastrointestinal se manifiesta por la aparición de disfagia orofaríngea, disfagia esofágica, reflujo gastroesofágico, gastroparesia, seudoobstrucción, sobrecrecimiento bacteriano y malabsorción intestinal, estreñimiento, diarrea y/o incontinencia fecal. Estas afectaciones condicionan la ingesta alimentaria y la absorción intestinal y conducen a la aparición progresiva de deficiencias nutricionales. Alrededor de un 30% de los pacientes con esclerosis sistémica presentan un riesgo de malnutrición. En el 5-10%, los trastornos gastrointestinales son la principal causa de muerte.
Las estrategias terapéuticas existentes en la actualidad son limitadas y están dirigidas a reducir la sintomatología clínica. El manejo multidisciplinar de dichos pacientes, que incluya la intervención nutricional, contribuye a mejorar la sintomatología gastrointestinal, además de evitar la malnutrición, la morbilidad y aumentar la calidad de vida.
In systemic sclerosis (SS), the second most frequently affected organ is the gastrointestinal tract (GIT) and particularly the esophagus.1 The clinical manifestations associated with the alteration of the GIT have malnutrition as a common denominator. A recent study by the Task Force of the Canadian Scleroderma (CSRG) Group showed that about 30% of an unselected sample of patients with SS had a moderate or high risk for malnutrition.2 Recently, a panel of U.S. experts has published a consensus on how to detect and manage malnutrition and GI disorders in SS.2 These are the leading cause of death in 5%–10% of patients with SS and GI involvement is associated with increased morbidity, mortality and reduced quality of life.3
The main pathological changes consist of GI smooth muscle atrophy and intestinal wall fibrosis, with dysphagia and gastroesophageal reflux being the most common digestive manifestations in SS.1,4
Oropharyngeal DysphagiaIt is due to oropharyngeal dysmotility, hindering the passage of the bolus from the mouth to the oropharynx.5 Patients may have skin involvement of the face and tongue, but these changes rarely affect oral4 intake. However, 20% of patients with SS have a associated Sjögren's syndrome.4 In this case, the reduction in saliva production is associated with oropharyngeal dysphagia due to the inability to properly lubricate the food bolus before being transferred into the esophagus. In addition, impairment of salivary secretion increases the risk of developing periodontal disease and accelerates tooth decay, making mastication difficult,6 so the evaluation of the oral cavity should be included routinely in the physical examination of such patients.3,4,7
Oropharyngeal dysphagia is characterized by occurring a few seconds after initiating the act of swallowing, and the feeling is manifested by retention of food in the throat and the need to swallow repeatedly.5 It is often accompanied by regurgitation of the bolus into the nasopharynx, which may expel food through the nose or larynx. Dysfunction of the constrictor muscles of the pharynx can cause dysarthria, nasal voice and pharyngonasal regurgitation.5,8 In more severe cases, the patient cannot swallow saliva and drools.5,8
Esophageal DysphagiaThe difficulty in swallowing arises once the bolus has passed through the pharynx and upper esophageal sphincter. This type of dysphagia may be due to a motor or sensitive mechanical disturbance.9
In SS, the overproduction of collagen leads to thickening and fibrosis. Muscles weaken and cause dysmotility, a mechanism that involves esophageal dysphagia.3,4 This alteration can range from a lack of coordination of esophageal motility to the existence of complete paralysis of the esophagus.1,3,4
Esophageal dysfunction in SS mainly occurs in the lower two-thirds of the esophagus, causing esophageal sphincter weakness and profound loss of peristaltic3 action. This dysfunction leads to reflux of stomach contents into the esophagus. Esophageal damage may manifest as a peptic ulcer or erosive esophagitis progressing to hemorrhage and ulceration.3 The ulcer may scar or progress to an esophageal stricture, fistula, achalasia and Barrett's esophagus with the risk of neoplasia.1,3,4
Dietary interventions not only depend on the degree of impairment but also must ensure adequate nutritional support, and are intended to modify the texture of foods, attaining adequate lubrication of food and a smooth consistency for each type of dysphagia, as well as reducing and/or preventing problems associated with itself.5,8,10–12 Dietary recommendations in cases of oropharyngeal dysphagia are shown in Table 1.5,8,11,12
Dietary Recommendations in Case of Swallowing Problems.
| Eat and chew slowly |
| Eat small amounts of food and often (5–6 meals a day) |
| To facilitate swallowing it is recommended that food to be consumed slightly cold |
| Drink water or other liquids between bites to aid swallowing |
| Carry a water bottle and drink in small sips of water throughout the day (6–8 glasses of water a day total) to ensure all bodily functions as well as production and quality of saliva |
| The consumption of sugar-free gum may help in some cases to stimulate the production of saliva and prevention of dental cavities |
| Soften food with oil, butter, margarine or sauces, especially meat and vegetables |
| Moisten dry foods like bread or crackers in liquid |
| Get the desired consistency by adding water, milk or broth or thickeners (cornstarch, flour, commercial infant cereal or prepared specific) |
| Recommended foods |
| Milk, yogurt, puddings, custards, cream, cheese |
| Ice creams, sorbets, puddings, mousses, soufflés… |
| Gelatin |
| Food with soft texture or can be crushed, pureed vegetables, pureed vegetables, omelets, scrambled eggs, fruit compote |
| Foods to avoid |
| Sticky foods such as boiled rice, sliced bread, mashed potatoes, banana… |
| Foods such as grilled meat, artichokes, asparagus… |
| Dry foods such as nuts, toast, potato “chip”, cookies… |
| Fish with bones and meat with small bones (rabbit) |
Gastroesophageal reflux is defined as the regurgitation of gastric contents into the esophagus and duodenum mainly due to loss of lower esophageal sphincter pressure 13. Recommendations are based on dietary changes and avoiding foods that promote reflux as well as reflux-inducing positions (Table 2).13
Dietary Recommendations in Cases of Gastroesophageal Reflux.
| Drink plenty of fluids (6–8 glasses a day) |
| Make small volume but frequent meals (5–6 a day) |
| Eat and chew slowly |
| Eat soft consistency or pureed foods |
| Avoid extremes |
| Avoid fat (butter, cream…) |
| Use simple cooking (grilled, baked, steamed, boiled) |
| Sit for 1–2h after meals, because gravity will aid peristalsis |
| Elevate the head of the bed and eat 2–3hours before bedtime |
| Avoid the following food |
| Alcohol |
| Carbonated beverages: glusoft drinks, cola… |
| Chocolate and derivatives |
| Caffeine (coffee, tea) |
| Wine, vinegar |
| Spices (pepper, mustard, cloves, oregano…) |
| Citrus fruits (orange, tangerine, lemon, grapefruit…) |
| Fermented cheeses (Manchego, Cabrales, Roquefort…) |
| Whole grains bread, brown rice, whole wheat pasta, whole grains, bran… |
| Garlic and onion |
| According to tolerance: cucumber, peppers and melons |
Esophageal stricture is a narrowing of the esophagus causing difficulty for swallowing. It is characterized by the appearance of a feeling of difficulty passing food or retrosternal pain right after swallowing, chest pain during eating and regurgitation of undigested food.4,8 Most patients are able to pinpoint the zone where this occurs.8
In this case, liquids are easier to swallow than solids. Virtually all foods can be liquefied, although for some of them it may be necessary to add a liquid (e.g. milk for grain, broth for meat, vegetables or fish). Dietary recommendations are presented in Table 3.8,11,12
Dietary Recommendations in Cases of Esophageal Stenosis.
| Liquid foods are easier to swallow than solids. Virtually any food or food can be liquefied. Grind or blend all possible foods to get a semi-solid or soft texture, adding any liquid or food support such as: cereal with milk, beef broth, vegetable broth with rice or vegetables, milk or water with fruit, cake and ice cream or sorbet … smoothie crushed texture should be smooth |
| Chew food thoroughly |
| Drink fluids to stay well hydrated (6–8 glasses a day) |
| Avoid dry foods like toast or biscuits, cookies, French fries or “chips”, snacks, nuts… |
| Avoid sticky foods like textured white bread, dried fruit, mashed potatoes, soft candy… |
| Avoid foods high in fiber or chips (bread, artichokes, asparagus…) |
| Avoid spicy foods spice and vinegar, and do not eat salty foods |
| Recommended semisolid food |
| Porridge |
| “Baby jars” or commercial purees |
| Meals baked egg souffle type |
| Cakes vegetables, meat or fish |
| Pudding, mousse, jello, pudding, custard, ice cream, sorbet |
| Fruit Compote |
Sometimes, despite performing a dietary intervention with conventional diet components, one may need to resort to commercial preparations or enteral14 nutrition.
Delayed Gastric Emptying or GastroparesisGastroparesis is a gastric motility disorder characterized by delayed emptying of solids and/or fluids from the stomach without any mechanical obstruction.15 Gastroparesis can worsen gastroesophageal reflux.3
Vascular ectasia is a cause of chronic gastrointestinal bleeding in patients with SS, who may experience a frank hemorrhage or bleeding causing microcytic or ferropenic anemia micro.3
Although no controlled trials evaluating the efficacy of dietary modification in the treatment of gastroparesis exist, and the clinical benefits are modest, some dietary recommendations may be useful in patients with milder forms of the disease.15
Nutritional intervention is based on dietary measures to encourage gastric emptying such as correct mastication, eliminating alcohol because it slows gastric emptying, fractionating a diet characterized by low but frequent volumes of food (5–6 meals a day), which may be low-fat and low in fiber to prevent bezoar formation. The soft consistency food or liquid is better tolerated. Fluid intake during a meal may promote gastric emptying. Orthostatic position or simply walking after eating in addition to other measures may facilitate gastric emptying. Nutritional therapy should be tailored to the severity of the disease. When weight loss occurs, the patient should be evaluated for the use of oral supplementation with enteral diets (complete diets in liquid form which need not be masticated or require stomach emptying). In later stages, when the stomach cannot empty liquids, enteral nutrition via a jejunostomy tube may be required, but parenteral nutrition while should be restricted to patients with severe gastric dysmotility of the small intenstine.7,15
Bowel InvolvementSS may affect both the small and large intestine, including the rectum and anus.
In the small intestine, dysmotility involves the immobilization of intraluminal contents, which promotes bacterial growth and consequent diarrhea.1,3,4 Pharmacological treatment with antacids also can support and/or aggravate bacterial2 overgrowth. All this contributes to impaired absorption of nutrients, causing malabsorption, which together with pseudoobstructions may lead to severe malnutrition.1–4,7
Complications including the colon are: luminal dilatation or pneumatosis, pseudo-obstruction, constipation, diarrhea, fecal incontinence and bleeding, often hidden by telangiectasia1 bleeding.3,4
In patients with severe manifestations of the small intestine and loss of response to antibiotics, one must consider artificial nutrition. An alternative may be nocturnal enteral nutrition, although in most cases it is more convenient and acceptable for the patient to undergo a percutaneous3 gastrostomy. The natural history of the disease may require parenteral3 nutrition.
- (1)
Constipation: due to intestinal4 dysmotility. In the early stages there appears to be effective constipation3 that can be initially treated with laxatives and soluble dietary fiber, which can also help improve fecal incontinence. Avoidance of non-absorbable carbohydrates such as sorbitol or lactulose is recommended as they may worsen the symptoms of bloating and discomfort, also favoring bacterial overgrowth.
- (2)
Diarrhea: dietary advice in this case has a limited effect, with the main objective being the reduction of clinical symptoms such as postprandial discomfort, bloating and loss of weight.4 The first step is to prescribe a diet low in fiber which may be easily digested and absorbed.
Restricting carbohydrates from the diet may reduce intestinal fermentation, as they are the main energy source of bacteria, and helps reduce symptoms.4 For this reason, the dietitian-nutritionist's diet should be tailored individually, based on progression, patient tolerance and habits.
Sometimes lactose intolerance appears, forcing its removal from the diet plan. Some studies have detected the anomalous presence of Lactobacillus or other probiotics in the small intestine, so their usefulness is questionable in these patients.4 In any case, patients with advanced stages of the disease may be candidates for artificial nutrition.
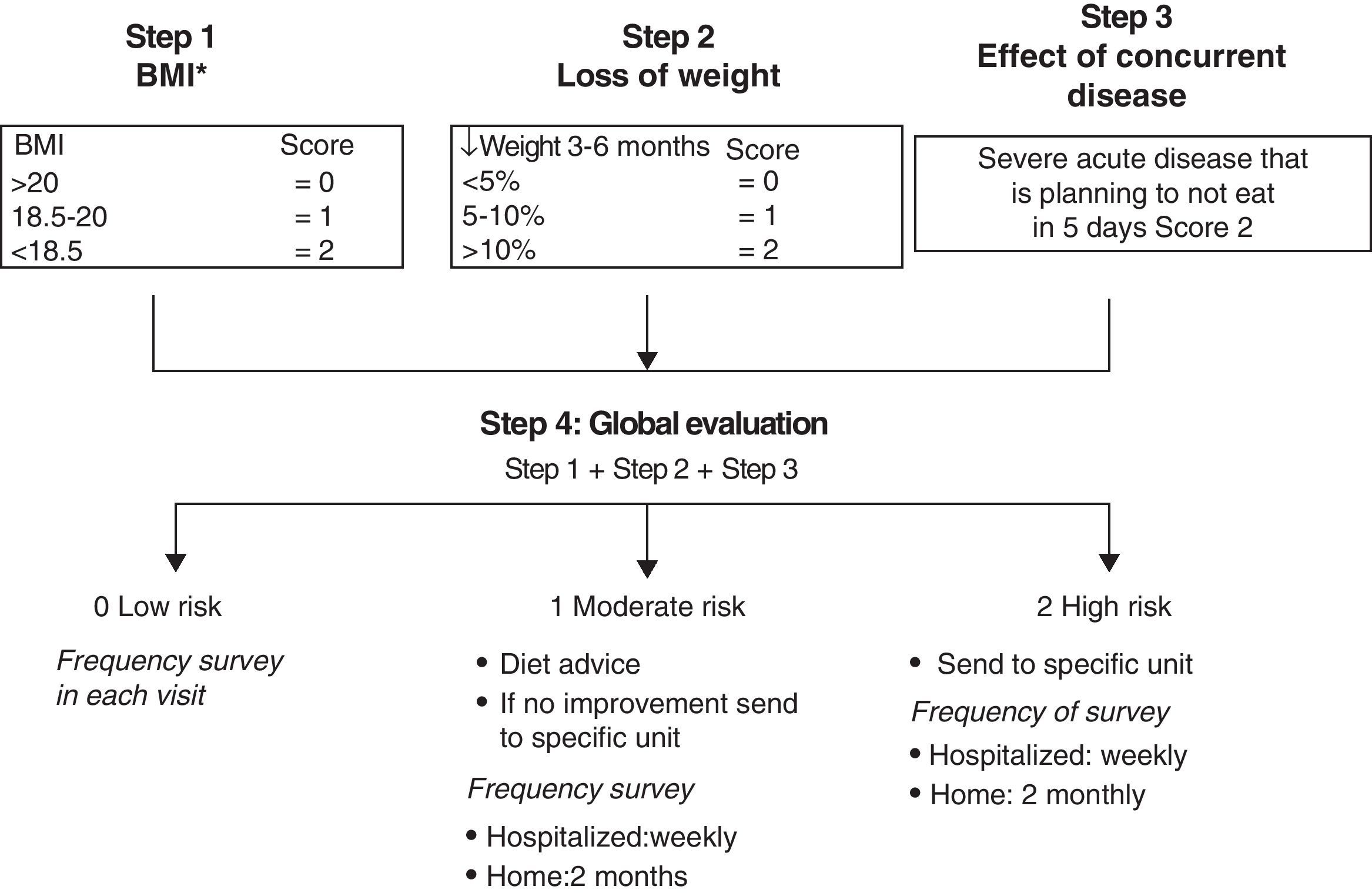
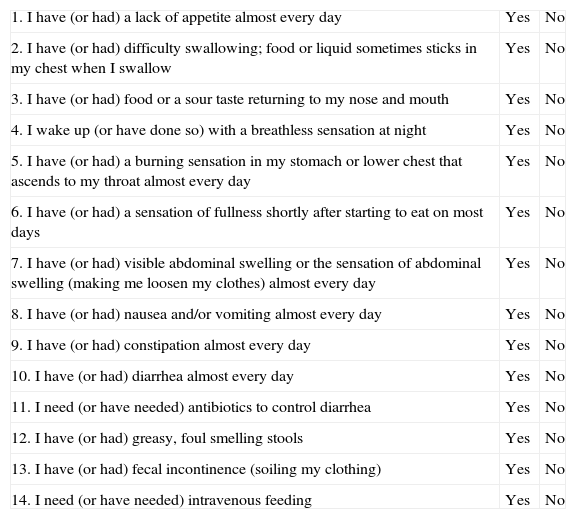
Methods for Screening for MalnutritionTo optimize resources and improve health care efficiency, questionnaires have been considered useful in clinical practice as a systematic way of screening to detect the involvement of different portions of the GIT. There are validated questionnaires developed by the CSRG2 (Table 4). To assess the risk of malnutrition,14 the Malnutrition Universal Screening Test (MUST)16 has already been used in this disease2 and is considered a practical tool for use in outpatient settings (Fig. 1).
Questionnaire Used by the Canadian Scleroderma Agency Research Group.
| 1. I have (or had) a lack of appetite almost every day | Yes | No |
| 2. I have (or had) difficulty swallowing; food or liquid sometimes sticks in my chest when I swallow | Yes | No |
| 3. I have (or had) food or a sour taste returning to my nose and mouth | Yes | No |
| 4. I wake up (or have done so) with a breathless sensation at night | Yes | No |
| 5. I have (or had) a burning sensation in my stomach or lower chest that ascends to my throat almost every day | Yes | No |
| 6. I have (or had) a sensation of fullness shortly after starting to eat on most days | Yes | No |
| 7. I have (or had) visible abdominal swelling or the sensation of abdominal swelling (making me loosen my clothes) almost every day | Yes | No |
| 8. I have (or had) nausea and/or vomiting almost every day | Yes | No |
| 9. I have (or had) constipation almost every day | Yes | No |
| 10. I have (or had) diarrhea almost every day | Yes | No |
| 11. I need (or have needed) antibiotics to control diarrhea | Yes | No |
| 12. I have (or had) greasy, foul smelling stools | Yes | No |
| 13. I have (or had) fecal incontinence (soiling my clothing) | Yes | No |
| 14. I need (or have needed) intravenous feeding | Yes | No |
The overall assessment of the nutritional status of patients with SS should include a survey on the food habits of the patient, ruling out any GI symptoms due to GI involvement (such as dysphagia, gastroparesis, nausea, vomiting, heartburn, rhythm disturbances…) through a validated questionnaire developed by the CSRG, and consider intercurrent disease.
On physical examination one must determine the weight, height, body mass index, and a complete inspection of the oropharyngeal cavity (teeth, chewing ability and salivation, the presence of infection), assessing the condition of the skin and mucosa to rule out nutritional deficiencies and the existence of edema.14
One may assess the risk of malnutrition by MUST and consider the evaluation of laboratory parameters. The basic analytical determinations established by the U.S. expert panel include: blood count, hemoglobin, vitamin A, folic acid, ferritin, vitamin B12.7 In the absence of folic acid supplementation, the detection of elevated plasma levels of this component may suggest the presence of bacterial overgrowth in the intestine. Low levels of vitamin A may be indicative of the existence of intestinal malabsorption, for which determination of zinc, 25-OH vitamin D and vitamin K levels and prothrombin7 time are recommended.
Although these patients generally have normal plasma albumin levels, and malnutrition is mostly caloric (marasmus), its determination in plasma may be useful for the diagnosis of mixed or protein-energy malnutrition (marasmus-kwashiorkor).
Depending on the symptoms and test results, one must consider assessing the need for additional tests (endoscopy, radiology tests, breath test, etc.).7,14
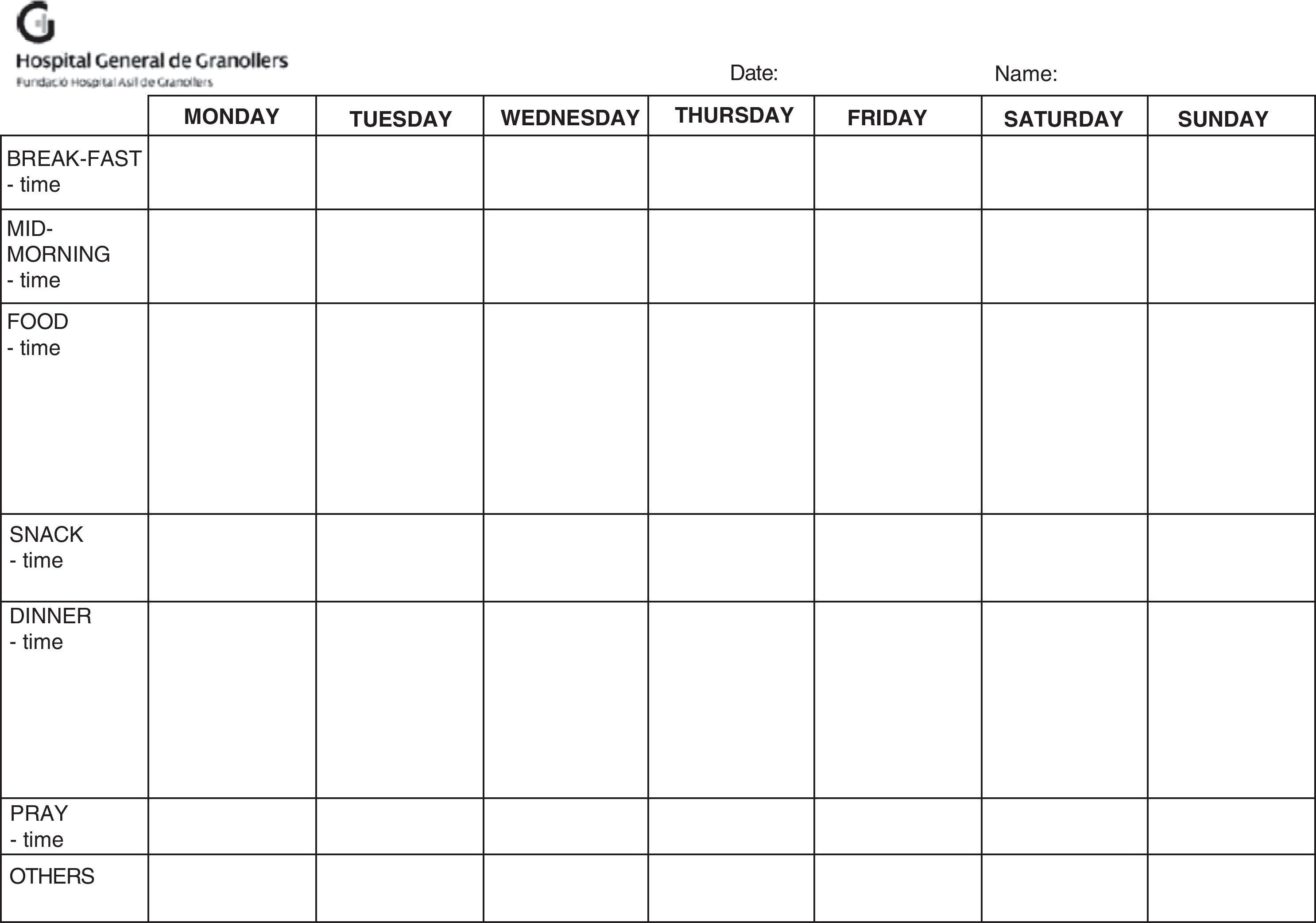
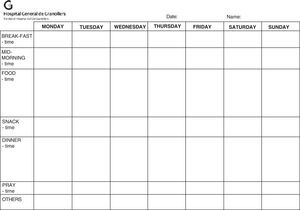
Dietary advice in writing should be provided and related to the pathology in question (Tables 1–3), and the need to refer the patient to the dietitian-nutritionist assessed, providing a food registry form to the patient (Fig. 2).
The involvement of multiple parts of the digestive tract and the development of highly personalized dietary recommendations are fundamental. In case the oral intake is insufficient with conventional foods, nutritional supplements are indicated orally.17 The indications for oral nutritional supplements and artificial nutrition do not differ from other chronic diseases.7,18
Follow-up is done by weighing the patient, as it constitutes the best indicator of malnutrition,7 and normalization of laboratory parameters in case of alterations.
ConclusionsAlterations of the GIT in patients with SS are very common. Throughout the entire gastrointestinal tract, manifestations are observed in relation to the concerned section. The common denominator of the GIT's condition is malnutrition. Malnutrition is associated with increased morbidity, mortality and reduced quality of life. Screening, detecting and early intervention by a multidisciplinary team, including nutritional intervention, are priorities in patients with SS.
Conflict of InterestThe authors declare that they have no conflicts of interest.
Please, cite this article as: Recasens MA, et al. Nutrición en la esclerosis sistémica. Reumatol Clin. 2012;8:135–40.