To evaluate the efficacy of tocilizumab (TCZ) in patients with rheumatoid arthritis (RA) in clinical practice, retention rates of the drug and predictors of response.
MethodsWe performed a descriptive, prospective, longitudinal, open-label study in patients receiving TCZ (8mg/kg/4 weeks) in a clinical practice setting. The clinical responses were evaluated using the European League Against Rheumatism (EULAR) response criteria, and the low activity and remission rates according to the Disease Activity Score 28-erythrocyte sedimentation rate (DAS28-ESR) and the Clinical Disease Activity Index (CDAI).
ResultsThe EULAR response rate was 86.63% and the DAS28 remission rate was 53.7% after 6 months of treatment; rates of low disease activity were 52.9% on CDAI and 47.1% on DAS28 at month 24.
There were no statistically significant differences in EULAR response, rates of low activity and remission on DAS28 between patients receiving TCZ alone and those receiving TCZ in combination therapy, or between patients positive or negative for rheumatoid factor (RF) and/or anti-cyclic citrullinated peptide (anti-CCP) antibodies. The naïve biological therapy patients showed better remission and low activity rates after 6 months of treatment. The retention rate was 61% at month 24. Adverse events were among the most frequent causes of discontinuation.
ConclusionsTocilizumab is effective in RA, has a similar efficacy when used alone or in combination with synthetic disease-modifying antirheumatic drugs (DMARDs) and shows high retention rates.
Evaluar la eficacia del tratamiento con tocilizumab (TCZ) en pacientes con artritis reumatoide (AR) en práctica clínica, las tasas de supervivencia del fármaco y variables clínicas predictoras de respuesta.
MétodosEs un estudio descriptivo, prospectivo, longitudinal y abierto en el que se incluyó a pacientes en condiciones de práctica clínica que recibieron TCZ (8mg/kg/cada 4 semanas). Las respuestas clínicas se midieron utilizando los criterios de respuesta de la European League Against Rheumatism (EULAR), las tasas de actividad baja y remisión según el Disease activity score-28 (DAS28-VSG) y Clinical Disease Activity Index (CDAI).
ResultadosLa tasa de respuesta EULAR fue del 86,63%, con una tasa de remisión DAS28 del 53,7% a los 6 meses de tratamiento. El 52,9% de los pacientes presentaron baja actividad de la enfermedad a los 24 meses según CDAI y 47,1% según DAS28. No hubo diferencias significativas en cuanto a respuesta EULAR, baja actividad y remisión DAS28 entre pacientes en tratamiento con TCZ en monoterapia y terapia combinada, ni entre pacientes positivos y negativos para factor reumatoide (FR) y/o anticuerpo antipéptido cíclico citrulinado (anti-PCC). Los pacientes que recibieron TCZ de primera línea presentaron mejores tasas de remisión y baja actividad a los 6 meses. La tasa de supervivencia fue del 61% a los 24 meses, siendo una de las causas de discontinuación más frecuente los efectos adversos.
ConclusiónEl TCZ es efectivo en pacientes con AR, tiene eficacia similar cuando se utiliza en monoterapia o en combinación con fármacos antirreumáticos modificadores de la enfermedad (FAME) sintéticos y presenta altas tasas de supervivencia.
Rheumatoid arthritis (RA) is a chronic inflammatory disease that mainly affects small and medium-sized joints. It is the most widely spread inflammatory arthritis, and is capable of producing substantial damage to the joints, as well as deformity and functional disability.1
The therapeutic objectives of the current recommendations in the management of RA patients focus on the complete remission of the disease. If that fails, its activity must be maintained at the lowest possible levels.2
The treatment of choice, once RA has been diagnosed, is a disease-modifying antirheumatic drug (DMARD). Methotrexate (MTX), unless contraindicated, is the recommended initial therapy.2
In the case of patients who have an unsatisfactory response to MTX or other DMARD, whether or not it is associated with glucocorticoids, the next therapeutic step consists in the administration of biological therapy in combination with a DMARD or alone. The available options include tumor necrosis factor (TNF) inhibitors, abatacept, tocilizumab (TCZ), anakinra and, in certain situations, rituximab.2,3
One of the therapeutic alternatives to be considered is TCZ, a humanized monoclonal antibody directed against the receptor, whether soluble or membrane-bound, of interleukin 6.4
The efficacy and safety of TCZ has been demonstrated in those patients with RA who have shown a satisfactory response to MTX/DMARD or TNF inhibitors in different clinical trials.5,6
The purpose of our study was to evaluate the outcome of our experience in terms of efficacy, as well as drug survival, and to analyze the causes for discontinuing the treatment in patients in routine clinical practice.
MethodsPatientsIn a descriptive, prospective, longitudinal, open-label study, we included patients diagnosed as having RA, according to the 1987 criteria of the American College of Rheumatology,7 who were treated with TCZ in a routine clinical setting between March 2009 and July 2015. These patients were recruited from the outpatient clinics of Teaching Hospital Virgen de la Nieves in Granada, in southeastern Spain. Seventeen of these patients had participated in a clinical trial: 10 in ACT-SURE5 and 7 in JUST-ACT.
The study group was constituted by 85 adults (over 18 years of age) who had been diagnosed with RA, in whom biological therapy was indicated according to the recommendations of the European League Against Rheumatism (EULAR)2 and the Spanish Society of Rheumatology.8,9
Tocilizumab was administered intravenously at a dose of 8mg/kg body weight every 4 weeks. The TCZ dose was adjusted in agreement with the technical specifications. Optimization of the treatment was not done in any patient. All of the participants gave their informed consent. The study was performed in accordance with the ethical principles of the Declaration of Helsinki and those consistent with good clinical practice.
VariablesThe baseline data of the patients (prior to starting treatment with TCZ) included: age, sex, disease duration, concomitant treatment with a DMARD, the number of biological agents used previously, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and blood parameters (hemoglobin, white blood cells, neutrophils and platelet count). The baseline serological findings for rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibodies were also recorded. At 6, 12 and 24 months, the following variables were collected: tender and swollen joint counts, visual analog scale (VAS) provided by the patient to assess pain and their disease, VAS on the disease provided by the physician, and blood test to determine ESR, RF, CRP and anti-CCP.
The primary objective of the study was to assess the efficacy of TCZ in our patients. It was evaluated using an intention-to-treat analysis, completed by patients who had received 1 or more doses of TCZ. Those who withdrew from the study were considered non-responders in the efficacy analysis.
We calculated the percentage of patients who achieved a significant clinical improvement according to the EULAR response criteria (reduction of ≥0.6 units in the disease activity score of 28 joints [DAS28] and a DAS28 ≤5.1), low disease activity (DAS28 ≤3.2) and remission (DAS28 <2.6) at 6, 12 and 24 months. Likewise, the rates of low disease activity and of clinical remission according to the Clinical Disease Activity Index (CDAI), with a score of ≤2.8 for remission and ≤10 for low activity, were also determined. Similarly, to identify any clinical variable associated with a better response to treatment, we compared the efficacy of TCZ in 3 patient subgroups: patients positive for RF and/or anti-CCP with respect to those who were negative for both variables, patients receiving TCZ as monotherapy with respect to patients receiving a concomitant DMARD (mainly MTX), and patients who had received a previous biological therapy versus those who had received no prior biological therapy.
Safety was evaluated by means of reports of adverse events (AE) leading to the discontinuation of the drug in patients who had received at least 1 dose of TCZ.
Drug survival was assessed throughout the 2 years of treatment, and those recorded for the abovementioned subgroups were compared.
Statistical AnalysisWe provide a descriptive analysis, with the determination of measures of central tendency and of dispersion for the numerical variables; absolute and relative frequencies were calculated for the categorical variables. The normal distribution of the data was assessed using the Shapiro-Wilks test.
Bivariate analysis was performed to evaluate the relationship of a response or disease activity to the use of a concomitant DMARD, number of previous biological therapies, positivity for RF and/or anti-CCP and the rest of the characteristics measured. For continuous variables, Student's t test was used for independent samples, or Mann–Whitney U test for cases in which the distribution was not normal. For categorical variables, we utilized the Pearson chi-squared test or Fisher's exact test.
Moreover, we did an analysis of drug survival using Kaplan–Meier's method to study the time to the end of treatment. The probabilities of survival were calculated, together with the 95% confidence interval, as well as the means and medians. The log-rank test was utilized to compare the groups (in terms of concomitant DMARD, previous biological therapies and positivity for RF and/or anti-CCP).
In all of the analyses, a P<.05 was considered to indicate significance. The data were analyzed using the IBM SPSS Statistics 19 software package.
ResultsPatientsThe study population included 85 patients, 72 (84.7%) of whom were women and 13 (15.3%) were men. They had a mean age of 54.92±11.36 years, a mean disease duration of 10.62±6.81 years, and a high disease activity (a mean DAS28 of 5.49±1.19 and mean CDAI of 26.96±13.12). Of the 85 patients, 38 received TCZ as monotherapy and 21 were taking TCZ as their first-line of treatment. The patients had previously received an average of 1.83±1.57 biological therapies and of 1.95±0.7 DMARD. Methotrexate was the most frequently associated DMARD (48.2%; n=41). In all, 68.7% of the patients were positive for RF and/or anti-CCP (Table 1).
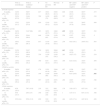
Baseline Clinical and Demographic Characteristics.
| Characteristic | Results (n=85) |
|---|---|
| Sex, men n (%)/women n (%) | 13 (15.3)/72 (84.7) |
| Age years, mean±SD | 54.92±11.36 |
| Duration of RA years, mean±SD | 10.62±6.8 |
| Number of previous biological treatments, mean±SD | 1.84±1.57 |
| Patients who received TCZ as first-line treatment, n (%) | 21 (24.7) |
| Patients who received TCZ as second-line treatment, n (%) | 19 (22.4) |
| Patients who received TCZ as third-line treatment, n (%) | 18 (21.2) |
| Patients who received TCZ as fourth-line treatment, n (%) | 14 (16.5) |
| Patients who received TCZ as fifth-line treatment, n (%) | 13 (15.3) |
| Number of previous DMARD, mean±SD | 1.95±0.7 |
| Patients receiving TCZ with methotrexate, n (%) | 41 (48.2) |
| Patients receiving TCZ with leflunomide, n (%) | 5 (5.9) |
| Patients receiving TCZ with sulfasalazine, n (%) | 1 (1.2) |
| Patients receiving TCZ as monotherapy, n (%) | 38 (44.7) |
| DAS28, mean±SD | 5.49±1.19 |
| CDAI, mean±SD | 26.96±13.1 |
| ESR, mean±SD | 36.07±22.3 |
| CRP, mean±SD | 1.93±2.7 |
| RF- and/or anti-CCP-positive, n (%) | 57 (67.1) |
| HAQ, mean±SD | 1.62±0.6 |
Anti-CCP, anti-cyclic citrullinated peptide antibodies; CDAI, Clinical Disease Activity Index; CRP, C-reactive protein; DAS28, Disease Activity Score based on 28 joints; DMARD, disease-modifying antirheumatic drugs; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; RA, rheumatoid arthritis; RF, rheumatoid factor; SD, standard deviation; TCZ, tocilizumab.
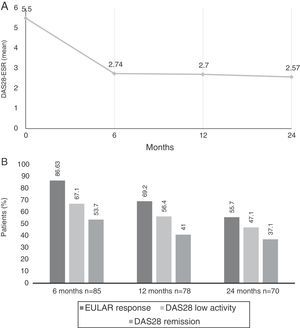
Comparing the baseline DAS28-ESR score, improvements were observed at each time point to up to 24 months (Fig. 1A). The mean change in the DAS28 after 6 months ((ΔDAS28) was −2.7±1.61, from −2.8 ± 1.57 at 12 months and from −3.0±1.66 at 24 months.
(A) Mean Disease Activity Score 28 joints-erythrocyte sedimentation rate (DAS28-ESR) at baseline visit and up to 2 years later. (B) Percentage of patients with DAS28 indicating low disease activity (≤3.2), remission (≤2.6) and European League Against Rheumatism (EULAR) response (decrease in DAS28 ≥0.6 and DAS28 ≤5.1) at 6, 12 and 24 months after beginning treatment with tocilizumab.
According to the EULAR, response rates were achieved in 86.6% (n=71) of the patients at 6 months, 69.2% (n=54) at 12 months and in 55.7% (n=39) at 24 months. At 6 months, 67.1% of the patients had low disease activity, and 53.7% were in clinical remission according to the DAS28; these rates were 56.4/41% at 12 months and 47.1/37.1% at 24 months, respectively (Fig. 1B).
According to the CDAI, 14.3% achieved remission at 6 months, 9.5% at 12 months and 11.4% at 24 months; however, 58.4% had low activity according to the CDAI at 6 months, 54.1% at 12 months and 52.9% at 24 months.
A decrease was recorded in the biological variables (CRP 0.52±1.15 and ESR 12.63±12.99) at 6 months, whereas they remained at normal levels throughout the 24 months of follow-up.
Analysis by SubgroupsEfficacy outcomes in the different patient subgroups are shown in Table 2.
Efficacy Outcomes in the Subgroups of Patients at 6, 12 and 24 Months After Initiating Tocilizumab Therapy.
| TCZ as monotherapy | TCZ in combined therapy | P | No previous BT | Previous BT | P | RF- and/or anti-CCP-negative | RF- and/or anti-CCP-positive | P | |
|---|---|---|---|---|---|---|---|---|---|
| EULAR response | |||||||||
| 6 months, n/No. (%) | 32/38 (84.2) | 39/47 (82.9) | .637 | 19/21 (90.5) | 52/64 (81.3) | .646 | 23/28 (82.1) | 48/57 (84.2) | .473 |
| 12 months, n/No. (%) | 23/34 (67.6) | 31/44 (70.5) | .790 | 14/20 (70) | 40/58 (68.9) | .797 | 16/27 (59.3) | 38/51 (74.5) | .218 |
| 24 months, n/No. (%) | 17/32 (53.1) | 22/38 (57.9) | .850 | 12/19 (63.2) | 27/51 (52.9) | .387 | 11/23 (47.8) | 28/47 (59.6) | .649 |
| DAS28 low activity | |||||||||
| 6 months, n/No. (%) | 24/38 (63.2) | 31/47 (66) | .923 | 18/21 (85.7) | 34/64 (53.1) | .028 | 16/28 (57.1) | 39/57 (68.4) | .583 |
| 12 months, n/No. (%) | 19/34 (55.9) | 25/44 (56.8) | .846 | 13/20 (65) | 31/58 (53.4) | .529 | 12/27 (44.4) | 32/51 (62.7) | .2 |
| 24 months, n/No. (%) | 16/32 (50) | 17/38 (44.7) | .837 | 11/19 (57.9) | 22/51 (43.1) | .279 | 9/23 (39.1) | 24/47 (51) | .643 |
| DAS28 remission | |||||||||
| 6 months, n/No. (%) | 21/38 (55.2) | 23/47 (48.9) | .530 | 16/21 (76.2) | 28/64 (43.8) | .041 | 12/28 (42.9) | 32/57 (56.1) | .28 |
| 12 months, n/No. (%) | 13/34 (38.2) | 19/44 (43.2) | .759 | 11/20 (55) | 21/58 (36.2) | .263 | 10/27 (37) | 22/51 (43.1) | .534 |
| 24 months, n/No. (%) | 13/32 (40.6) | 13/38 (34.2) | .790 | 8/19 (42.1) | 18/51 (35.3) | .459 | 8/23 (34.8) | 18/47 (38.3) | .959 |
| CDAI low activity | |||||||||
| 6 months, n/No. (%) | 20/38 (52.6) | 25/47 (53.2) | .178 | 13/21 (61.9) | 32/64 (50) | .057 | 13/28 (46.4) | 32/57 (56.1) | .170 |
| 12 months, n/No. (%) | 22/34 (64.7) | 18/44 (40.9) | .198 | 12/20 (60) | 28/58 (48.3) | .559 | 8/27 (29.6) | 32/51 (62.7) | .006 |
| 24 months, n/No. (%) | 19/32 (59.4) | 18/38 (47.4) | .557 | 12/19 (63.2) | 25/51 (49) | .294 | 10/23 (43.5) | 27/47 (57.4) | .546 |
| CDAI remission | |||||||||
| 6 months, n/No. (%) | 4/38 (10.5) | 7/47 (14.9) | .218 | 3/21 (14.2) | 8/64 (12.5) | .178 | 3/28 (10.7) | 8/57 (14) | .397 |
| 12 months, n/No. (%) | 5/34 (14.7) | 2/44 (4.5) | .356 | 2/20 (10) | 5/58 (8.6) | 1 | 1/27 (3.7) | 6/51 (11.8) | .308 |
| 24 months, n/No. (%) | 3/32 (9.4) | 5/38 (13.2) | .820 | 3/19 (15.8) | 5/51 (9.8) | .444 | 4/23 (17.4) | 4/47 (8.5) | .636 |
Anti-CCP, anti-cyclic citrullinated peptide antibodies; BT, biological therapy; CDAI, Clinical Disease Activity Index; CDAI low activity, CDAI ≤10; CDAI remission, CDAI ≤2.8; DAS28, Disease Activity Score based on 28 joints; DAS28 low activity, DAS28 ≤3.2; DAS28 remission, DAS28<2.6; EULAR (European League Against Rheumatism) response, decrease in DAS28 ≥0.6 and DAS28 ≤5.1; RF, rheumatoid factor; TCZ, tocilizumab.
Significant P values in boldface.
When compared with patients receiving TCZ monotherapy, with respect to those who were taking combined therapy, it was observed that, of the 38 patients being treated with TCZ alone, 32 (84.2%) demonstrated EULAR response criteria at 6 months, 67.6% at 12 months and 53.1% at 24 months. This contrasts with the 82.9% at 6 months, 70.5% at 12 months and 57.9% at 24 months, of those patients who were receiving a concomitant DMARD, showing differences that were not statistically significant.
Likewise, 63.2% (24/38) of the patients receiving treatment with TCZ as monotherapy met the criteria for low activity according to the DAS28 at 6 months, as did 55.9% (19/34) at 12 months and 50% (16/32) at 24 months; among the patients with combined therapy, the rates were 66%, 56.8% and 44.7% at 6, 12, and 24 months, respectively.
There were no statistically significant differences with respect to the DAS28 remission rates between the patients taking TCZ monotherapy (55.2%, 38.2% and 40.6%, at 6, 12 and 24 months, respectively) and those receiving treatment with combined therapy (48.9%, 43.2% and 34.2%, at 6, 12 and 24 months).
At 6 months, only 10.5% of the patients with TCZ as monotherapy achieved remission according to the CDAI, with 14.7% at 12 months and 9.4% at 24 months; this compares with 14.9% at 6 months, 4.5% at 12 months and 13.2% at 24 months, among those receiving combined therapy.
We found no significant differences between the 2 groups in terms of the rates of low activity according to the CDAI (52.6%, 64.7% and 59.3% in patients receiving monotherapy, and 53.2%, 40.9% and 47.4% in those taking combined therapy, at 6, 12 and 24 months).
Biological Therapy-naïve PatientsOf the patients who had received no previous biological treatment, 90.5% at 6 months, 70% at 12 months and 63.2% at 24 months, showed EULAR response criteria, and in those who had taken at least 1 previous biological agent, these rates were 81.3%, 68.9% and 52.9% at 6, 12 and 24 months, respectively, differences that did not reach statistical significance.
In all, 85.7% of the patients who were naïve to biological therapy had low activity rates, according to the DAS28 at 6 months, as did 65% at 12 months and 57.9% at 24 months. The rates of remission in terms of the DAS28 were 43.8% at 6 months of treatment in patients who had taken at least 1 biological DMARD previously, and were 36.2% at 12 months and 35.2% at 24 months. A comparison between these groups showed that the differences were statistically significant for the rates of low activity and for remission after 6 months of treatment (P=.028 and P=.041).
With regard to the rates of low activity and of remission according to the CDAI, the patients who had received no prior biological DMARD had higher rates throughout the study, although the differences were not statistically significant.
Rheumatoid Factor and/or Anti-cyclic Citrullinated Peptide AntibodiesThere were no differences between the patients who were positive for RF and/or anti-CCP with respect to those who were RF- and anti-CCP-negative in terms of the EULAR response, with regard to low activity or clinical remission, except in the rates of low activity according to the CDAI at 12 months, in which 62.7% of the patients positive for RF and/or anti-CCP had low activity according to the CDAI at 12 months (P=.006).
SafetyThe safety outcomes that resulted in the discontinuation of the treatment are shown in Table 3.
Safety Outcomes: Adverse Events That Can Result in the Discontinuation of Tocilizumab.
| No.=85 | |
|---|---|
| AE, n (%) | 16 (18.82) |
| Oral ulcers, n (%) | 1 (1.17) |
| Reactions to the injection, n (%) | 5 (5.88) |
| Urinary tract infections, n (%) | 1 (1.17) |
| Respiratory tract infections, n (%) | 2 (2.35) |
| Gastrointestinal tract involvement, n (%) | 2 (2.35) |
| Neutropenia, n (%) | 2 (2.35) |
| Malignant neoplasm, n (%) | 1 (1.17) |
| Intestinal perforation, n (%) | 1 (1.17) |
| Deaths, n (%) | 1 (1.17) |
AE, adverse events.
In all, TCZ was interrupted in 16 patients due to AE. Infections were among the most frequent AE (n=5), as were reactions to the injection, which occurred in 5 patients. Two patients developed neutropenia, although the neutrophil count never fell below 500/mm,3 and 1 patient had an intestinal perforation. One patient developed an ovarian adenocarcinoma, and there was 1 death (a stroke) during the study, not related to the treatment.
In relation to the increase in liver enzymes and total cholesterol, it was not necessary to discontinue the administration of TCZ, since it was controlled either by adjusting the TCZ dose or with a specific treatment.
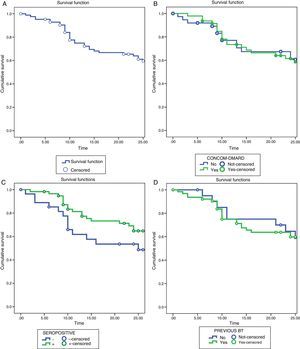
Tocilizumab Retention RatesThe retention rates with TCZ were 72.2% at 12 months and 61% at 24 months. The reasons for discontinuing the treatment were: lack of efficacy (16 patients, 48.5%), AE (16 patients, 48.5%) and other causes that do not include patients in remission (1 patient, 3%).
We found no statistically significant differences when comparing the rates of survival or the reasons for withdrawing from the treatment in the different subgroups of patients (Fig. 2).
Survival analysis using the Kaplan–Meier method. (A) Drug survival of TCZ during 24 months of treatment. (B) Survival of TCZ as monotherapy vs combined therapy. (C) Survival of TCZ in patients positive for RF and/or anti-CCP vs RF- and/or anti-CCP-negative patients. (D) Survival of TCZ in BT-naïve vs patients with previous BT. Anti-CCP, anti-cyclic citrullinated peptide antibodies; BT, biological therapy; DMARD, disease-modifying antirheumatic drug; RF, rheumatoid factor; TCZ, tocilizumab.
Tocilizumab has been shown in different clinical trials to be effective in reducing the activity of the disease and in inhibiting the radiological progression in patients with RA.10–14
The improvement in the parameters of disease activity and remission according to the DAS28 achieved in 53.7% of the patients after 6 months are similar to those reported in the TAMARA study, in which the rate of remission after 24 weeks was 47.6%.15
The higher percentage of patients in remission according to the DAS28, in comparison to the application of the CDAI, could be related to the effect of TCZ on acute-phase reactants and the influence of the ESR on the DAS28 formula.16 However, the fact that the low activity rates are similar in both the CDAI and the DAS28 would explain that the efficacy of TCZ is not only related to its effect on acute-phase reactants.
Considering clinical remission according to the CDAI, 14.3% achieved values indicating remission after 6 months, which was reached by 11.4% at 24 months. This could be associated with the discrepancy in the perception of disease activity on the part of patients and physicians; as patients need to perceive greater improvement and less deterioration to achieve some degree of satisfaction.17
The results obtained have not indicated significant differences in the clinical effectiveness of TCZ as monotherapy when compared with treatment in combination with a synthetic DMARD. These findings are similar to those of other clinical trials.18–22
In patients who have never received biological therapy, according to the DAS28, remission is achieved in 80% at 6 months. These results are more supportive of the data from the ACT-SURE study, in which the patients who were naïve to anti-TNF therapy had higher response rates with TCZ than the patients who had been treated previously with an anti-TNF agent.23
The available literature demonstrates the fact that patients who have not been previously treated with a biological therapy have better response rates.24,25 In our study, comparing the efficacy among that group of patients with that of patients who had already been treated with a biological DMARD, the differences observed were statistically significant, after only 6 months of treatment, not over the long term. These data would agree with those of Kaneko et al.,26 in which the efficacy according to the DAS28 and CDAI is compared in the 2 groups, with significant differences only at the initiation of the treatment.
In association with the data on safety, the frequency of discontinuing treatment with TCZ due to an AE was 18.8% in our study, which is slightly higher than the 10.1% recently observed in a study by Iking-Konert et al., which could be associated with the method of data collection in that report, which was not done directly.27
The retention rates in our study were 72.2% at 12 months, similar to those of the REACTION study, where the rate of survival at week 52 was 71.1%.28 This did not differ among patients who had received TCZ in monotherapy or in combination with a synthetic DMARD. These results contrast with the findings of Gabay et al.,29 who observed that the retention rate of TCZ was higher when it was prescribed in combination with MTX, reaching statistical significance at 1.5 years of treatment, and that the main reason for withdrawing was the loss of efficacy. In our study, the survival rate after 2 years was slightly greater in those patients who were taking TCZ as monotherapy, although without significant differences between the groups. The differences found in our study and others based on patient registries, concerning the rate of retention of TCZ, when utilized in monotherapy or in combined therapy, could be the reflection of differences in the availability of the drug, local recommendations, economic situations or the availability of other treatment options, and even preferences for prescribing a given treatment on the part of the physician.
Within the limitations of this study, we include those associated with an observational study, in that it has no control group and, as it involves only a single center, the number of patients is reduced. Although, we evaluated the efficacy of TCZ in our routine clinical practice, we included 17 patients who had participated in a clinical trial; after analyzing the baseline demographic variables of these patients, and comparing them with that of our patients in the clinical practice, we concluded that they were similar and, thus, they were included in the study (data are not shown).
In conclusion, our study demonstrates that treatment with TCZ, both in combination and as monotherapy, is effective in patients with longstanding RA. It produces a sustained reduction in the biological parameters and shows a high rate of retention. More studies should be performed in the clinical practice setting, including more patients, and thus enabling us to demonstrate our results.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Notario Ferreira I, Ferrer González MA, Morales Garrido P, González Utrilla A, García Sanchez A, Soto Pino MJ, et al. Eficacia a 2 años de tocilizumab en pacientes con artritis reumatoide activa en la práctica clínica habitual. Reumatol Clin. 2017;13:78–84.