Interstitial lung disease is a leading cause of mortality in patients with systemic sclerosis. Currently, there is a lack of consensus regarding screening, rescreening, diagnosis, and follow-up practices in interstitial lung disease associated with systemic sclerosis (SSc-ILD) in Colombia.
MethodsA structured survey focused on clinical practices in patients with SSc-ILD was conducted. Members of the Asociación Colombiana de Neumología y Cirugía de Tórax (Asoneumocito) and the Asociación Colombiana de Reumatología (Asoreuma) were invited to participate from March 2023 to May 2023.
ResultsWe surveyed 51 pulmonologists and 44 rheumatologists. Overall, 51.6% reported having access to multidisciplinary team discussion in ILD. Among the 95 participants, 78.9% would routinely perform a high-resolution computed tomography scan of the chest once a diagnosis of systemic sclerosis was established. This practice is more frequent among rheumatologists (84.1%) than among pulmonologists (74.5%). Approximately half of the participants would rescreen patients annually with computed tomography scan (56.8%) if baseline images were negative.
Spirometry (81.1%), diffusing capacity of the lung for carbon monoxide (80.0%), and 6-min walk test (55.8%) were the most frequently performed tests upon diagnosis of systemic sclerosis. During follow-up, participants would consider repeating pulmonary function tests mostly every 6 months.
ConclusionsScreening of SSc-ILD is high among pulmonologists and rheumatologists. Decision-making on diagnosis and follow-up is similar between specialties, but there are variations in their frequency and indications. Further research is needed to evaluate how to adapt recommendations for assessing SSc-ILD in different settings.
La enfermedad pulmonar intersticial (EPI) es una de las principales causas de mortalidad en pacientes con esclerosis sistémica. Actualmente, existe una falta de consenso sobre las prácticas de cribado, recribado, diagnóstico y seguimiento de la EPI asociada a esclerosis sistémica (SSc-EPI) en Colombia.
MétodosSe realizó una encuesta estructurada, centrada en las prácticas clínicas de pacientes con SSc-EPI. Se invitó a participar a miembros de la Asociación Colombiana de Neumología y Cirugía de Tórax (ASONEUMOCITO) y la Asociación Colombiana de Reumatología (ASOREUMA) desde marzo de 2023 hasta mayo de 2023.
ResultadosSe encuestaron 51 neumólogos y 44 reumatólogos. En general, 51,6% informó tener acceso a equipos de discusión multidisciplinaria en EPI. Entre los 95 participantes, 78,9% realizaría de forma rutinaria una tomografía computarizada de tórax de alta resolución una vez hecho el diagnóstico de esclerosis sistémica. Esta práctica es más frecuente entre los reumatólogos (84,1%) que entre los neumólogos (74,5%). Aproximadamente, la mitad de los participantes reexaminaría a los pacientes anualmente con una tomografía computarizada (56,8%) si las imágenes iniciales fueran negativas.
La espirometría (81,1%), la capacidad de difusión pulmonar de monóxido de carbono (80,0%) y la caminata de seis minutos (55,8%) fueron las pruebas realizadas con mayor frecuencia tras el diagnóstico de esclerosis sistémica. Durante el seguimiento, los participantes considerarían repetir las pruebas de función pulmonar principalmente cada seis meses.
ConclusionesEl cribado de SSc-EPI es común entre neumólogos y reumatólogos. La toma de decisiones sobre el diagnóstico y seguimiento es similar entre especialidades, pero existen variaciones en su frecuencia e indicaciones. Es necesario investigar cómo adaptar las recomendaciones de evaluación de la SSc-EPI a diferentes entornos.
Systemic sclerosis (SSc) is a heterogeneous autoimmune disease characterized by microvascular damage and generalized fibrosis that can have potential major complications depending on the internal organ involvement.1 Interstitial lung disease (ILD) is one of the most frequent complications, with a prevalence ranging from 44% to 50%.2 ILD invariably leads to decreased lung function, worsening symptoms, impaired quality of life, high healthcare costs, and early mortality.1,3 Individuals with advanced age, longer duration of SSc, diffuse SSc subtype, decreased baseline forced vital capacity (FVC), positive anti-topoisomerase I (anti-Scl70) antibodies, negative anti-centromere antibodies or raised inflammatory markers have an increased risk of SSc-ILD.2,4
Considering the high prevalence of ILD in SSc patients, systematic screening for SSc-ILD, especially in those at high risk, is supported by consensus-based recommendations.5–7 Because of its sensitivity, high-resolution computed tomography (HRCT) of the chest is recommended for screening.6,8 Pulmonary function tests (PFT) have lower sensitivity for diagnosing ILD, but they support diagnosis, assess severity, and diagnose progression at follow-up.8,9
Progression of SSc-ILD predicts mortality; some of its risk factors are male sex, higher modified Rodnan skin score, extent of ILD on HRCT and gastroesophageal reflux disease.4,10 In the EUSTAR database, 23–27% of patients with SSc-ILD showed progression in each 12-month follow-up period; however, the behavior of progression is highly heterogeneous and may vary in consecutive follow-up periods.11,12
Assessment of ILD needs a multidisciplinary approach of clinical, functional, imaging, and histopathological data to integrate a clinical probability for a diagnosis.13 However, due to limited knowledge of SSc, barriers to access to diagnostic tests, and late referral to specialists, many cases are misdiagnosed, increasing morbidity and mortality.14,15 This situation has been documented in Latin America, where availability of PFT, diagnostic imaging, and ILD referral centers is low.16
In Colombia, there is a lack of clinically relevant data and expert-based consensus to diagnose and manage ILDs, including SSc-ILD. It is likely that barriers to healthcare access promote heterogenous practices in the different regions of the country for the diagnosis, follow-up, and treatment of these diseases.17 Therefore, we conducted a national survey to assess the current practice for the diagnosis and follow-up of SSc-ILD patients by pulmonologists and rheumatologists in Colombia.
MethodsStudy design and participantsWe designed an online survey on screening, diagnosis, and follow-up practices in SSc-ILD patients (Supplementary file). Considering that there are no validated questions aimed at assessing these issues, we reviewed the medical literature to develop an instrument that would respond to the objective of the study, focused on the Colombian context. The electronic questionnaire was sent to the Asociación Colombiana de Neumología y Cirugía de Tórax (Asoneumocito) and the Asociación Colombiana de Reumatología (Asoreuma) members, aimed at practicing pulmonologists and rheumatologists in Colombia with experience in the treatment of SSc-ILD. Awareness was raised through reminders to obtain the highest possible participation rate.
The survey was organized in 23 structured questions with multiple-choice answers and branching logic, based on how participants answered specific questions. The survey was composed of four main sections: participants’ background, availability of diagnostic testing and access to multidisciplinary team discussion, clinical practices on SSc-ILD screening and diagnosis, and diagnosis of SSc-ILD progression. Participants were asked to rate the importance of some variables in the assessing risk of progression using a five-point Likert scale (1=Not at all important; 2=Slightly important; 3=Moderately important; 4=Very important; 5=Extremely important).
Progress through the questionnaire was not allowed until the participant completed each question. The survey was tested before it was fielded in a small group of pulmonologists and rheumatologist to assess wording and completion time. It was open for responses from March 2023 to May 2023.
Statistical analysisOnly completed questionnaires were analyzed. Categorical data are expressed as number and percentage of respondents. For continuous data, mean with standard deviation (SD) or median with interquartile range (IQR) are presented, as appropriate.
Survey data was collected and processed using the electronic data capture tools of the REDCap (Research Electronic Data Capture) platform version 13.4.10 (Vanderbilt University, Nashville, TN, USA). Analyses were performed using R statistical software version 4.3.1 (R Core Team, 2023).
Ethical considerationsPhysicians were invited to participate without any monetary or non-monetary incentive. All data provided by the participants was voluntary and confidential. Data of each survey were anonymized.
The survey was accompanied by a brief text with information about the content and the purpose of the survey, the investigators involved, and the approximate length of time to completion.
Physicians involved in the design and analysis were excluded from participating in the survey.
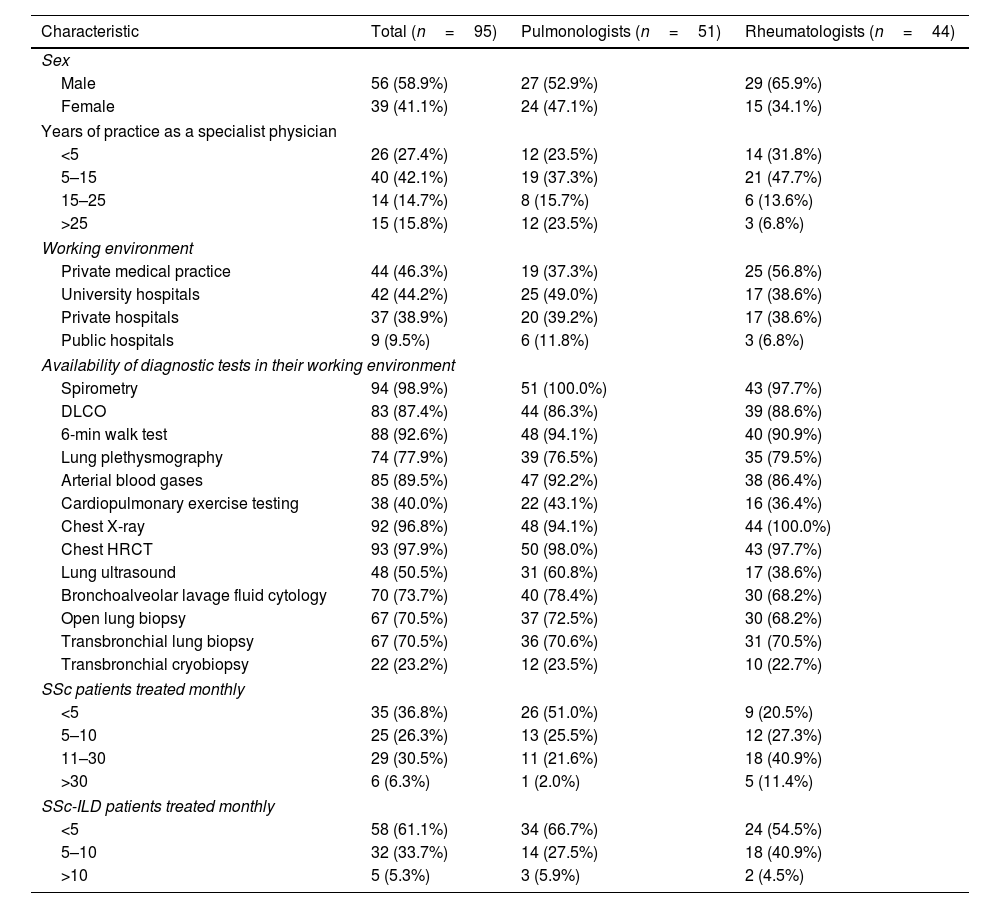
ResultsParticipant characteristicsA total of 95 (51 pulmonologists and 44 rheumatologists) out of 432 invited physicians (178 pulmonologists and 254 rheumatologists) responded to the survey (22.0% response rate). The participants were individuals with a median age of 43 years (IQR 39.0–52.8), mainly from major cities of Colombia (39 from Bogotá [41.1%], 16 from Cali [16.8%], 9 from Medellín [9.5%], 4 from Barranquilla [4.2%], 4 from Bucaramanga [4.2%]).
Pulmonologists and rheumatologists practiced predominately in hospital-based clinics (university hospitals, private hospitals, and public hospitals), some of them across multiple care settings (Table 1). Their collective practice as a specialist physician was generally five years or more (72.6%), with no differences between both specialties. Rheumatologists reported treating more SSc patients monthly compared to pulmonologists, but this difference was smaller regarding the number of patients with SSc-ILD.
Characteristics of the participants.
| Characteristic | Total (n=95) | Pulmonologists (n=51) | Rheumatologists (n=44) |
|---|---|---|---|
| Sex | |||
| Male | 56 (58.9%) | 27 (52.9%) | 29 (65.9%) |
| Female | 39 (41.1%) | 24 (47.1%) | 15 (34.1%) |
| Years of practice as a specialist physician | |||
| <5 | 26 (27.4%) | 12 (23.5%) | 14 (31.8%) |
| 5–15 | 40 (42.1%) | 19 (37.3%) | 21 (47.7%) |
| 15–25 | 14 (14.7%) | 8 (15.7%) | 6 (13.6%) |
| >25 | 15 (15.8%) | 12 (23.5%) | 3 (6.8%) |
| Working environment | |||
| Private medical practice | 44 (46.3%) | 19 (37.3%) | 25 (56.8%) |
| University hospitals | 42 (44.2%) | 25 (49.0%) | 17 (38.6%) |
| Private hospitals | 37 (38.9%) | 20 (39.2%) | 17 (38.6%) |
| Public hospitals | 9 (9.5%) | 6 (11.8%) | 3 (6.8%) |
| Availability of diagnostic tests in their working environment | |||
| Spirometry | 94 (98.9%) | 51 (100.0%) | 43 (97.7%) |
| DLCO | 83 (87.4%) | 44 (86.3%) | 39 (88.6%) |
| 6-min walk test | 88 (92.6%) | 48 (94.1%) | 40 (90.9%) |
| Lung plethysmography | 74 (77.9%) | 39 (76.5%) | 35 (79.5%) |
| Arterial blood gases | 85 (89.5%) | 47 (92.2%) | 38 (86.4%) |
| Cardiopulmonary exercise testing | 38 (40.0%) | 22 (43.1%) | 16 (36.4%) |
| Chest X-ray | 92 (96.8%) | 48 (94.1%) | 44 (100.0%) |
| Chest HRCT | 93 (97.9%) | 50 (98.0%) | 43 (97.7%) |
| Lung ultrasound | 48 (50.5%) | 31 (60.8%) | 17 (38.6%) |
| Bronchoalveolar lavage fluid cytology | 70 (73.7%) | 40 (78.4%) | 30 (68.2%) |
| Open lung biopsy | 67 (70.5%) | 37 (72.5%) | 30 (68.2%) |
| Transbronchial lung biopsy | 67 (70.5%) | 36 (70.6%) | 31 (70.5%) |
| Transbronchial cryobiopsy | 22 (23.2%) | 12 (23.5%) | 10 (22.7%) |
| SSc patients treated monthly | |||
| <5 | 35 (36.8%) | 26 (51.0%) | 9 (20.5%) |
| 5–10 | 25 (26.3%) | 13 (25.5%) | 12 (27.3%) |
| 11–30 | 29 (30.5%) | 11 (21.6%) | 18 (40.9%) |
| >30 | 6 (6.3%) | 1 (2.0%) | 5 (11.4%) |
| SSc-ILD patients treated monthly | |||
| <5 | 58 (61.1%) | 34 (66.7%) | 24 (54.5%) |
| 5–10 | 32 (33.7%) | 14 (27.5%) | 18 (40.9%) |
| >10 | 5 (5.3%) | 3 (5.9%) | 2 (4.5%) |
HRCT: high-resolution computed tomography; DLCO: diffusing capacity of the lung for carbon monoxide; SSc: systemic sclerosis; SSc-ILD: interstitial lung disease associated with systemic sclerosis.
Overall, 37.9% of the participants claimed to have access to a radiologist with an emphasis in thoracic radiology in the working environment where they work most of the time; 28.4% have access to a pathologist with an emphasis in pulmonary pathology. Only 51.6% of the participants reported having access to multidisciplinary team discussion in their clinical practice.
SSc-ILD screening and rescreenMost respondents routinely screen ILD with HRCT of the chest in all asymptomatic and newly diagnosed SSc patients (78.9%), but this practice differs between medical specialties, with rheumatologists screening more than pulmonologists (84.1% vs. 74.5%, respectively). Other participants perform a HRCT only if there is dyspnea (at rest or during exercise) or cough (29.5%), which is more frequent among pulmonologists compared to rheumatologists (35.3% vs. 22.7%, respectively). Other reasons for performing a HRCT of the chest were if FVC is <70% of the predicted value or diffusing capacity of the lung for carbon monoxide (DLCO) is <80% of the predicted value (23.2%), if there are fine crackles on auscultation (21.1%), if there is a higher risk antibody profile (16.8%), or if there is high-risk skin involvement (diffuse cutaneous SSc) (11.6%).
Among those who do not routinely screen with HRCT, their main reasons for not doing so are that they believe there is not enough scientific evidence in this setting (5.3%), followed by having ethical concerns (radiation exposure) (4.2%), concerns about economic cost (4.2%), or because they rely more on clinical suspicion (2.1%).
When asked about rescreening if the baseline HRCT of the chest was negative for ILD, more than half of the participants would routinely perform a HRCT annually (56.8%), but there was significant variation between specialties in this practice, with rheumatologists rescreening more compared to pulmonologists (65.9% vs. 49.0%, respectively). Other reasons for repeat HRCT of the chest were new onset or worsening dyspnea (at rest or during exercise) or cough (69.5%), FVC or DLCO decline (66.3%), new onset fine crackles on auscultation (57.9%), or new onset or worsening hypoxemia (at rest or during exercise) (55.8%). The decision of pulmonologists to repeat HRCT, compared to rheumatologist, is mainly driven by the presence of FVC or DLCO decline (76.5% vs. 54.5%, respectively) or by new onset or worsening hypoxemia (62.7% vs. 47.7%, respectively).
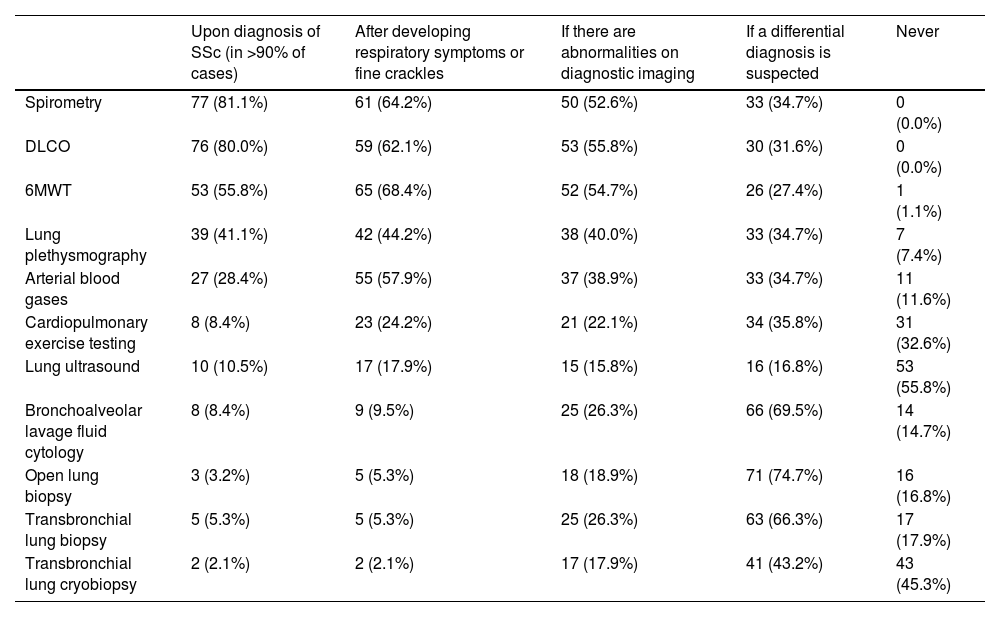
SSc-ILD diagnosisParticipants estimated that the time between the onset of respiratory symptoms and diagnosis of SSc-ILD is 13 months (IQR 12–24).
Spirometry (81.1%), DLCO (80.0%), and 6-minute walk test (6MWT) (55.8%) were the most performed PFTs upon diagnosis of SSc. These tests are also frequently performed if patients develop respiratory symptoms or fine crackles on auscultation (spirometry 64.2%, DLCO 62.1%, and 6MWT 68.4%) or if there are abnormalities on diagnostic imaging (spirometry 52.6%, DLCO 55.8%, and 6MWT 54.7%) (Table 2).
Indications for testing for the diagnosis of SSc-ILD.
| Upon diagnosis of SSc (in >90% of cases) | After developing respiratory symptoms or fine crackles | If there are abnormalities on diagnostic imaging | If a differential diagnosis is suspected | Never | |
|---|---|---|---|---|---|
| Spirometry | 77 (81.1%) | 61 (64.2%) | 50 (52.6%) | 33 (34.7%) | 0 (0.0%) |
| DLCO | 76 (80.0%) | 59 (62.1%) | 53 (55.8%) | 30 (31.6%) | 0 (0.0%) |
| 6MWT | 53 (55.8%) | 65 (68.4%) | 52 (54.7%) | 26 (27.4%) | 1 (1.1%) |
| Lung plethysmography | 39 (41.1%) | 42 (44.2%) | 38 (40.0%) | 33 (34.7%) | 7 (7.4%) |
| Arterial blood gases | 27 (28.4%) | 55 (57.9%) | 37 (38.9%) | 33 (34.7%) | 11 (11.6%) |
| Cardiopulmonary exercise testing | 8 (8.4%) | 23 (24.2%) | 21 (22.1%) | 34 (35.8%) | 31 (32.6%) |
| Lung ultrasound | 10 (10.5%) | 17 (17.9%) | 15 (15.8%) | 16 (16.8%) | 53 (55.8%) |
| Bronchoalveolar lavage fluid cytology | 8 (8.4%) | 9 (9.5%) | 25 (26.3%) | 66 (69.5%) | 14 (14.7%) |
| Open lung biopsy | 3 (3.2%) | 5 (5.3%) | 18 (18.9%) | 71 (74.7%) | 16 (16.8%) |
| Transbronchial lung biopsy | 5 (5.3%) | 5 (5.3%) | 25 (26.3%) | 63 (66.3%) | 17 (17.9%) |
| Transbronchial lung cryobiopsy | 2 (2.1%) | 2 (2.1%) | 17 (17.9%) | 41 (43.2%) | 43 (45.3%) |
6MWT: 6-min walk test; DLCO: diffusing capacity of the lung for carbon monoxide.
Pulmonologists, compared to rheumatologists, are more likely to perform spirometry (84.3% vs. 77.3%), DLCO (82.4% vs. 77.3%), 6MWT (66.7% vs. 43.2%) and arterial blood gases (41.2% vs. 13.6%) upon diagnosis of SSc. Likewise, pulmonologists are more likely to perform spirometry (68.6% vs. 59.1%), DLCO (64.7% vs. 59.1%), 6MWT (70.6% vs. 65.9%) and arterial blood gases (66.7% vs. 47.7%) if patients develop respiratory symptoms or fine crackles on auscultation.
Bronchoalveolar lavage (69.5%), open lung biopsy (74.7%), transbronchial lung biopsy (66.3%), and transbronchial lung cryobiopsy (43.2%) are considered mainly if a differential diagnosis is suspected. Participants estimated that only 5% (IQR 0–20) of their SSc-ILD patients have a lung biopsy.
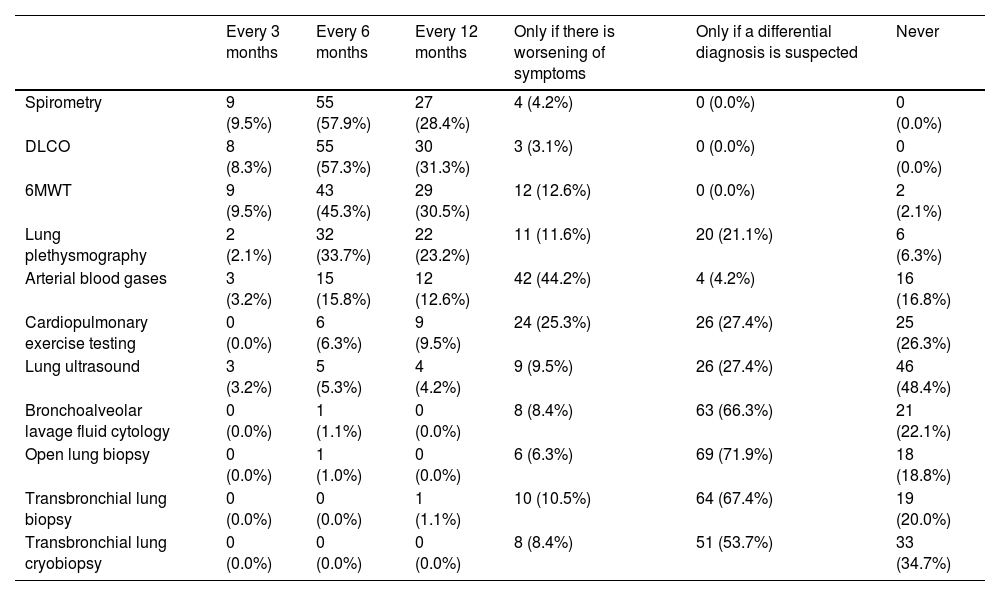
Follow-up of SSc-ILD patients and diagnosis of progressionDuring follow-up, participants consider repeating PFTs mainly every 6 months (spirometry 57.9%, DLCO 56.8%, and 6MWT 45.3%) (Table 3). Pulmonologists prefer to schedule spirometry every 6 months compared to rheumatologists (66.7% vs. 47.7%, respectively), while rheumatologists schedule it every 12 months compared to pulmonologists (47.7% vs. 11.8%, respectively); this clinical practice is similar for DLCO, which pulmonologists prefer to perform every 6 months (64.7% vs. 47.7%, respectively), while rheumatologists prefer to perform every 12 months (47.7% vs. 17.6%, respectively). Arterial blood gases are mainly repeated only if symptoms worsen (44.2%).
Indications for testing in the follow-up of patients with SSc-ILD.
| Every 3 months | Every 6 months | Every 12 months | Only if there is worsening of symptoms | Only if a differential diagnosis is suspected | Never | |
|---|---|---|---|---|---|---|
| Spirometry | 9 (9.5%) | 55 (57.9%) | 27 (28.4%) | 4 (4.2%) | 0 (0.0%) | 0 (0.0%) |
| DLCO | 8 (8.3%) | 55 (57.3%) | 30 (31.3%) | 3 (3.1%) | 0 (0.0%) | 0 (0.0%) |
| 6MWT | 9 (9.5%) | 43 (45.3%) | 29 (30.5%) | 12 (12.6%) | 0 (0.0%) | 2 (2.1%) |
| Lung plethysmography | 2 (2.1%) | 32 (33.7%) | 22 (23.2%) | 11 (11.6%) | 20 (21.1%) | 6 (6.3%) |
| Arterial blood gases | 3 (3.2%) | 15 (15.8%) | 12 (12.6%) | 42 (44.2%) | 4 (4.2%) | 16 (16.8%) |
| Cardiopulmonary exercise testing | 0 (0.0%) | 6 (6.3%) | 9 (9.5%) | 24 (25.3%) | 26 (27.4%) | 25 (26.3%) |
| Lung ultrasound | 3 (3.2%) | 5 (5.3%) | 4 (4.2%) | 9 (9.5%) | 26 (27.4%) | 46 (48.4%) |
| Bronchoalveolar lavage fluid cytology | 0 (0.0%) | 1 (1.1%) | 0 (0.0%) | 8 (8.4%) | 63 (66.3%) | 21 (22.1%) |
| Open lung biopsy | 0 (0.0%) | 1 (1.0%) | 0 (0.0%) | 6 (6.3%) | 69 (71.9%) | 18 (18.8%) |
| Transbronchial lung biopsy | 0 (0.0%) | 0 (0.0%) | 1 (1.1%) | 10 (10.5%) | 64 (67.4%) | 19 (20.0%) |
| Transbronchial lung cryobiopsy | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 8 (8.4%) | 51 (53.7%) | 33 (34.7%) |
6MWT: 6-min walk test; DLCO: diffusing capacity of the lung for carbon monoxide.
HRCT of the chest is infrequently repeated annually to detect progression of ILD (44.2%). Participants consider repeat HRCT of the chest upon certain indications including FVC or DLCO decline (80.0%), new onset or worsening dyspnea (at rest or during exercise) or cough (80.0%), new onset or worsening hypoxemia (at rest or during exercise) (71.6%) or to assess the effects of treatment (53.7%). Pulmonologists consider repeating HRCT mainly if there is FVC or DLCO decline compared to rheumatologists (88.2% vs. 70.5%, respectively).
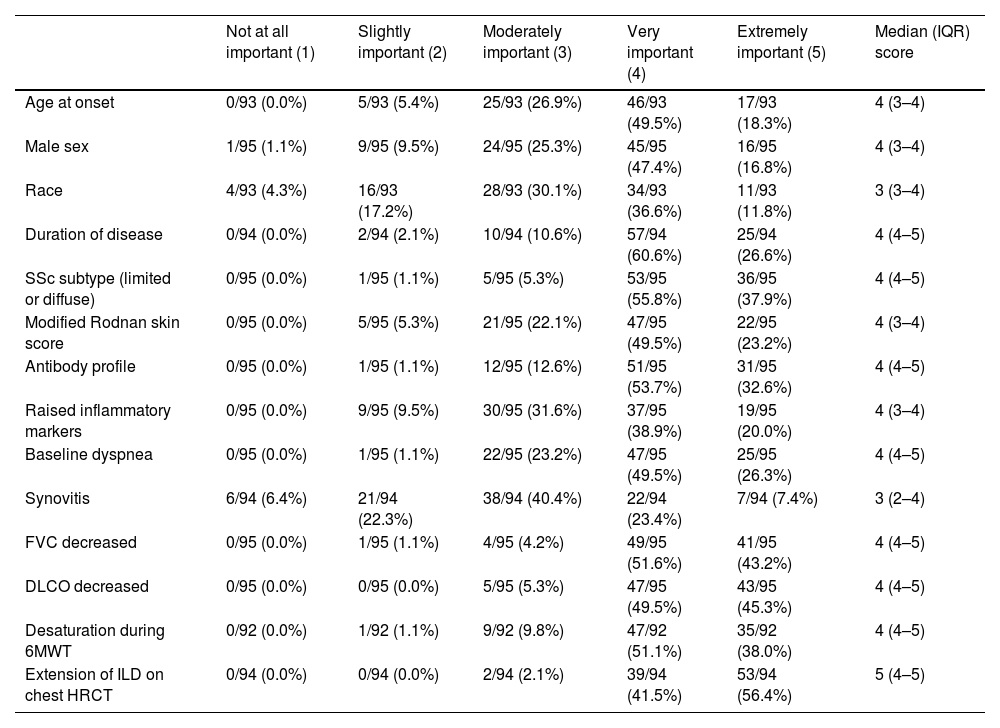
The participants estimated that 30% (IQR 20–50) of the SSc-ILD patients they evaluate progressed despite treatment in the last year. Assessment of risk factors for progression, such as the extent of SSc-ILD on HRCT of the chest, decreased FVC, decreased DLCO, desaturation during 6MWT, baseline dyspnea, antibody profile, duration of SSc and SSc subtype (limited or diffuse) were considered extremely important or very important (Table 4).
Assessment of the importance of some risk factors for the development of SSc-ILD progression.
| Not at all important (1) | Slightly important (2) | Moderately important (3) | Very important (4) | Extremely important (5) | Median (IQR) score | |
|---|---|---|---|---|---|---|
| Age at onset | 0/93 (0.0%) | 5/93 (5.4%) | 25/93 (26.9%) | 46/93 (49.5%) | 17/93 (18.3%) | 4 (3–4) |
| Male sex | 1/95 (1.1%) | 9/95 (9.5%) | 24/95 (25.3%) | 45/95 (47.4%) | 16/95 (16.8%) | 4 (3–4) |
| Race | 4/93 (4.3%) | 16/93 (17.2%) | 28/93 (30.1%) | 34/93 (36.6%) | 11/93 (11.8%) | 3 (3–4) |
| Duration of disease | 0/94 (0.0%) | 2/94 (2.1%) | 10/94 (10.6%) | 57/94 (60.6%) | 25/94 (26.6%) | 4 (4–5) |
| SSc subtype (limited or diffuse) | 0/95 (0.0%) | 1/95 (1.1%) | 5/95 (5.3%) | 53/95 (55.8%) | 36/95 (37.9%) | 4 (4–5) |
| Modified Rodnan skin score | 0/95 (0.0%) | 5/95 (5.3%) | 21/95 (22.1%) | 47/95 (49.5%) | 22/95 (23.2%) | 4 (3–4) |
| Antibody profile | 0/95 (0.0%) | 1/95 (1.1%) | 12/95 (12.6%) | 51/95 (53.7%) | 31/95 (32.6%) | 4 (4–5) |
| Raised inflammatory markers | 0/95 (0.0%) | 9/95 (9.5%) | 30/95 (31.6%) | 37/95 (38.9%) | 19/95 (20.0%) | 4 (3–4) |
| Baseline dyspnea | 0/95 (0.0%) | 1/95 (1.1%) | 22/95 (23.2%) | 47/95 (49.5%) | 25/95 (26.3%) | 4 (4–5) |
| Synovitis | 6/94 (6.4%) | 21/94 (22.3%) | 38/94 (40.4%) | 22/94 (23.4%) | 7/94 (7.4%) | 3 (2–4) |
| FVC decreased | 0/95 (0.0%) | 1/95 (1.1%) | 4/95 (4.2%) | 49/95 (51.6%) | 41/95 (43.2%) | 4 (4–5) |
| DLCO decreased | 0/95 (0.0%) | 0/95 (0.0%) | 5/95 (5.3%) | 47/95 (49.5%) | 43/95 (45.3%) | 4 (4–5) |
| Desaturation during 6MWT | 0/92 (0.0%) | 1/92 (1.1%) | 9/92 (9.8%) | 47/92 (51.1%) | 35/92 (38.0%) | 4 (4–5) |
| Extension of ILD on chest HRCT | 0/94 (0.0%) | 0/94 (0.0%) | 2/94 (2.1%) | 39/94 (41.5%) | 53/94 (56.4%) | 5 (4–5) |
6MWT: 6-min walk test; FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide; HRCT: high-resolution computed tomography; ILD: interstitial lung disease; SSc: systemic sclerosis.
This survey is the first study that provides an overview of how specialists assess and monitor patients with SSc-ILD in the Colombian context, describing screening, rescreening, diagnosis, and follow-up practices. While the availability of diagnostic tests, including PFTs and imaging, in the working environment of the participants is high, access to specialized radiologists or pathologists and multidisciplinary team discussion is low, despite the fact that integrated approach in multidisciplinary teams is the gold standard for diagnosis of ILDs.18
Most patients with SSc-ILD are respiratory asymptomatic, especially when the disease is mild, so close follow-up is important as clinical behavior could be unpredictable.11,12 Interdisciplinary expert consensuses recommend baseline HRCT in all SSc patients for ILD screening, with greater agreement for patients with respiratory symptoms and those with high-risk factors, as PFTs have low sensitivity for early detection of ILD.5–7 In our study, screening with HRCT of the chest is performed by 78.9% of the participants, which is high compared to previous surveys. Nevertheless, there are disparities in diagnostic approaches between pulmonologists and rheumatologists due to uncertainty among physicians about how to perform an appropriate evaluation. Some participants rely more on clinical risk factors, symptoms, or functional impairment to order a HRCT.
In a global survey conducted in 2020 only 64.9% of respondents screened for ILD with HRCT in patients with newly diagnosed SSc, while 30.7% ordered HRCT only on clinical suspicion (fine crackles on auscultation, FVC<80% of the predicted value, FVC or DLCO decline, or dyspnea).19 It seems that the screening rate has increased due to consensuses recommendations, as a previous survey had described that only 51% of general rheumatologists and 66% of SSc experts routinely ordered HRCT in newly diagnosed SSc patients.20
We realized that one of the participants’ reasons for not performing screening HRCT included the lack of sufficient clinical evidence beyond expert-based recommendation. To favor clinical decision making on routine screening, further studies should evaluate its impact and cost-effectiveness in different settings. Another potential drawback of HRCT is the radiation risk; tools such as lung ultrasound, low-dose or reduced number of slices HRCT protocols may be helpful in a screening and rescreening algorithm, although they need to be validated and cannot replace HRCT as the imaging of choice for the diagnosis of ILD.21–23
Despite there are no recommendations on rescreening, 56.8% of our participants would repeat a HRCT annually, especially rheumatologists. Pulmonologists rely more on symptoms and functional worsening. In the last global survey, only 14.1% of the participants would perform an annual HRCT in the rescreening, since they do so more on clinical suspicion (64.9%).19 Decisions in this aside should probably be guided by risk factors for developing SSc-ILD and new onset of symptoms or functional impairment, balancing healthcare costs and impact on SSc prognosis.
In our study, participants scheduled PFTs (spirometry and DLCO) mainly every 6 months, but rheumatologists prefer a longer interval for follow-up. Currently, it is suggested to monitor the progression of fibrosing ILDs with PFTs at least every 3–4 months during the first year, whereas serial HRCT is often required on a case-by-case basis, less frequently than clinical and functional assessment.24 Repeat HRCT of the chest annually to detect progression was a less common decision for our participants (44.2%) compared with repeat PFTs. Although HRCT may be a more sensitive method than spirometry for monitoring disease progression in early SSc-ILD, there is no consensus regarding HRCT intervals of repetition.6,25 Repeat imaging can be performed according to the individual patient's risk factors for progression or if complementary information to PFTs is needed, with an annual follow-up interval or less frequently if the patient is clinically stable or improving.24
Participants of our survey estimated that 30% of their patients progress over a year, which is consistent with what has been reported.11 Progression of ILD can be identified by combinations of increasing respiratory symptoms, PFTs decline, and increasing fibrosis on HRCT, with no alternative explanation for worsening.24,26 For ILD secondary to autoimmunity, OMERACT (Outcome Measures in Rheumatology) has proposed to standardize the concept of clinically significant progression by a relative decline in FVC of ≥10% or by a relative decline in FVC of ≥5% to <10% and a relative decline in DLCO of ≥15%.27 In SSc-ILD, progression is proposed to be defined by involvement in more than one of the following domains: spirometry and gas exchange, respiratory symptoms, and quantitative HRCT.28 Some of the individual predictors of poor outcomes in SSc-ILD may help prioritize populations requiring closer monitoring; composite measures could be used in clinical practice to design follow-up algorithms.29,30 Our participants chose the extent of ILD on HRCT, baseline PFTs impairment, desaturation during 6MWT, baseline dyspnea, antibody profile, duration of SSc and SSc subtype as the main factors they considered in assessing progression.
There are embedded limitations in survey research. Participants were not selected by random sampling; this may lead to a volunteer effect among participants and a lack of representativeness that may not accurately reflect the opinions and characteristics of the broader population of physicians treating SSc-ILD. Since more than half of the participants treat less than five SSc-ILD patients per month, we may have excluded more experienced specialists due to convenience sampling. However, given that the participants were members of the only two associations of pulmonologists and rheumatologists in Colombia, they could be representative of the population subset where the findings are applicable. Although we used reminders to increase the participation rate there was a low response, so the conclusions drawn from this sample are limited. The design of the questionnaire could have caused bias, since the questions selected could have affected how participants evaluated their own clinical practice. To manage for response bias, the questionnaire consisted mostly of closed multiple-choice questions, so participants possibly did not have to speed through it to finish it quickly. We tested the survey to be completed in 10–15min.
The reasons why each pulmonologist or rheumatologist is more likely to make any specific clinical decision in the SSc-ILD setting can’t be explained by the design of this study and should be explored by qualitative research. The results of this survey may help to generate hypotheses for further studies and to adapt or develop consensus recommendations in the specific context of Colombia.7
In conclusion, the results of this survey provide insights into the real-life practice of pulmonologists and rheumatologists in the screening and monitoring of SSc-ILD in Colombia. Our findings help to identify areas with a lack of consensus by both specialists and provide directions for future research. Strategies should be tailored and designed based on local data to meet health needs and support clinical decision making to homogenize approaches of care.
Authors’ contributionsStudy conceptualization and design: all authors. Data collection: all authors. Statistical analysis: JLG, OMG. Interpretation of results: all authors. Manuscript preparation: all authors. All authors read and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare that they have no conflict of interests regarding the publication of this article.
To the Asociación Colombiana de Neumología y Cirugía de Tórax (Asoneumocito) and the Asociación Colombiana de Reumatología (Asoreuma) members for their participation in the survey.