Given the lack of standardized tools to assess tolerability from patient's perspective, we carried out the validation of a questionnaire to asses treatment tolerability for rheumatoid arthritis (RA): TOL-AR-18.
MethodsThe TOL-AR-18 was validated in the cross-sectional observational study in adult patients with RA. Socio-demographic and clinical variables were collected. Patients completed the TOL-AR-18 and ARTS questionnaires and a question about their general health status.
ResultsData of 66 patients with a mean age (standard deviation; SD) of 56.71 (10.50) years were analyzed; 66.7% were women. Mean (SD) time to disease progression was 10.76 (8.23) years, and time from first treatment initiation was 9.24 (7.70) years. Patients did not report other adverse events rather than those included in the TOL-AR-18. The questionnaire was feasible with high internal consistency (Cronbach's α >0.90). Discriminant validity was moderate, with significant differences between patients in remission and all other, and between patients who did not report adverse events and the rest (P<.05). Convergent validity was moderate–low when correlated with the ARTS questionnaire.
ConclusionThe TOL-AR-18 is valid for use in a clinical setting and useful as an additional tool for all professionals to manage and assess tolerability in RA.
La ausencia de herramientas estandarizadas para evaluar la tolerabilidad desde la perspectiva del paciente, planteó la posibilidad de validar el cuestionario TOL-AR-18 de tolerabilidad al tratamiento de la artritis reumatoide (AR) y evaluar su aplicación en la práctica clínica.
MétodosEl desarrollo del cuestionario se realizó siguiendo la metodología estandarizada y posteriormente se validó mediante un estudio observacional y transversal con pacientes >18 años diagnosticados de AR, en tratamiento durante al menos 3 meses. Se recogieron variables socio-demográficas y clínicas. Los pacientes completaron los cuestionarios TOL-AR-18, ARTS, y el cuestionario sobre el estado de salud percibida.
ResultadosSe analizaron datos de 66 pacientes de edad media (desviación estándar [DE]) de 56,71 (10,50) años; un 66,7% eran mujeres y el 65,1% presentaba remisión/baja actividad por DAS28. La media (DE) del tiempo de evolución y del inicio del primer tratamiento fue de 11,34 (9,41) y 9,24 (7,7) años, respectivamente. Los pacientes no reportaron reacciones adversas adicionales a las incluidas en el TOL-AR-18. El cuestionario resultó factible con una consistencia interna elevada (α de Cronbach>0,9). La validez discriminante fue moderada, con diferencias estadísticamente significativas entre pacientes en remisión y el resto, y entre quienes no reportaban reacciones adversas y el resto (p<0,05). La validez convergente fue moderada-baja al correlacionarse con el cuestionario ARTS.
ConclusionesEl cuestionario TOLAR-18 es válido para su uso en la práctica clínica y útil como herramienta complementaria para el manejo y valoración de la tolerabilidad en la AR por parte de todos los profesionales implicados.
The prognosis of chronic inflammatory diseases such as rheumatoid arthritis (RA) has improved substantially in the last decade with the introduction of biological therapies, but despite the demonstrated efficacy and effectiveness of these treatments, toxicity is also an important factor to take into account.1 Up to 20%–40% of patients treated with methotrexate (MTX) have been observed to experience tolerability problems requiring drug withdrawal. Adverse events (AEs) are among the factors that most limit the use of some treatments and determine the management of patients in daily clinical practice.2
Many studies analyze the tolerability of pharmacological therapies for RA, but traditionally it is examined only from a clinical point of view and in overall terms (using treatment drop-out/discontinuation rates, persistence rates, laboratory tests or brief interviews with patients during a visit), without taking into account the impact of symptom or events treatment-related on patient's life. From the patient's perspective, tolerability includes detection of treatment-related events, but also assessment of their impact on daily life. For example, fatigue may not be a very important or critical AE from a clinical perspective, but it may cause a high degree of disability from the patient's point of view in terms of professional activities. These patient-reported outcomes (PROs) are becoming increasingly essential in clinical trials conducted in patients with chronic diseases, observational studies and, ultimately, daily clinical practice. PROs include a part of the patient's life that is not assessed at medical visits and may provide information that affects therapeutic decisions, such as suspension of a drug.3–5
Some studies have noted that, in general, clinicians report a substantially lower number of AEs than patients do,6,7 and that patients tend to provide a more detailed description, including information about its impact on, for example, health-related quality of life (HRQoL).8 In several diseases such as schizophrenia and bipolar disorder, specific questionnaires to assess the impact of secondary effects on HRQoL have begun to be validated and used.9,10 The few studies on RA that include the patient's assessment of tolerability do so using non-standardized tools with ad-hoc questions for patients or with HRQoL questionnaires that are not designed to assess tolerability.11,12 The patient's perspective is a concept that is gaining such importance that some countries such as the United Kingdom, the United States, Australia and Sweden have added reports supplied by patients to their pharmacovigilance systems.13,14
This fact, as well as the lack of validated tools, arouses the interest of authors to conduct a study with the objective of designing and validating a questionnaire to assess tolerability in patients under treatment for RA (TOL-AR-18) and to describing the potential utility of the questionnaire to assess tolerability of treatment for rheumatoid arthritis in the daily clinical practice of rheumatologists and other professionals involved in the management of these patients. The results of the validation are presented below.
Materials and MethodsQuestionnaire Design (Summary of the Stages)The study fulfilled the Spanish regulatory requirements and informed consent for observational studies according to the Ethics Commite from “Fundació de Recerca guidelines from Hospital Universitari Parc Taulí de Sabadell, Barcelona”. All patients includded signed the informed consent form to participate.
Before validation, the TOL-AR-18 questionnaire was designed using a standardized methodology for development of patient-centred measures15 with four steps:
Establishment of Conceptual Framework and Literature ReviewA bibliographic review was made in PUBMED of the published literature on questionnaires and tolerability evaluation studies in RA combining terms related to the pathology, with the type of results to be evaluated (tolerability, satisfaction, quality of life related to health (HRQOL)) and with different treatments. The search was limited to studies published in English and Spanish for the last 5 years, although it had to be extended to the last 10 years due to the few articles identified. Of the 388 bibliographic references obtained, a first screening was performed, eliminating those that were considered not to perform an assessment of tolerability from the patient's point of view or those studies that compared effectiveness between pharmacological treatments, obtaining only 5 publications that They could be considered useful for the purpose. Then after, a manual search was conducted when 4 more references were identified, based on reviews of the literature and mainly related to adherence. With the reading of the selected articles, it was verified that there is currently no published questionnaire elaborated with standardized methodology and it became evident the scarcity of studies in which the tolerability is evaluated, not in purely clinical terms but from the perspective of the patients. This review, however, was useful in identifying the adverse reactions most frequently reported by patients.
Focus Group With 6 Expert Rheumatologists in RA to Select the Most Common AEs, Related Health Dimensions and Most Typical Manifestations in PatientsWith a list of 27 symptoms, adverse reactions and discomforts related to the treatment for RA obtained from the review of the literature, a focus group was carried out with a group of 6 rheumatologists, experts in RA, in order to select those most frequent complaints and the health dimensions mostly affected by each one. During the focus group three exercises were carried out:
- a)
Review of the list of symptoms and reactions: the experts, by consensus, included, excluded or grouped those items that they considered according to their clinical experience, selecting those that were related to the treatments and more usually reported by the patients.
- b)
Relation of each of the expressions or items obtained from the previous exercise with the health dimension with which it could be more linked or that could be more affected (physical, psychological, social, cognitive, sexual, daily activities, perception of health, energy-vitality and pain.
- c)
Evaluation of the degree of importance and frequency of each item with a scale of 0 (not frequent/important) to 4 (very frequent/important). From this stage a first list of 19 items was obtained, those that were above the 50 percentile in importance and frequency and above the 75 percentile in the average of both. With those items, a first version of the questionnaire was obtained and the format and response options were designed.
Seven patients were interviewed in order to ensure the understanding of the statements, the response categories and the instructions on the part of the patients. The interviews were conducted by the same interviewer to minimize interviewer bias. The interviews were structured as follows: First, patients were given the first version of the “Tolerability questionnaire for rheumatoid arthritis treatment” without receiving any clarification from the interviewer. The interviewer then conducted a semi-structured interview with the patients to assess the understanding of the statements and the response system. In addition, for each item, they were asked if they thought that reaction or symptom was due to the treatment they were taking for RA or if they understood it as a symptom of the disease.
Qualitative Analysis of the Interviews and Obtaining the Final Items of the Questionnaire to be Validated in Phase IIOnce the transcripts of the interviews were finished, a qualitative analysis of their content was carried out by 3 experts in the development of questionnaires. The criteria used to modify the content of the questionnaire was that at least 2 patients expressed the need to change some aspect or content of the questionnaire. After this analysis it was decided to exclude only the item: “I have an altered appetite (Increase/decrease)” since the patients related it more to the increase/loss of weight, aspect that was collected in previous items, than with the appetite directly.
With the changes made in this analysis, the final questionnaire of 18 items was obtained object of its subsequent validation.
The TOL-AR-18 questionnaire consists of 18 dichotomic items (yes/no) about the presence of 18 typical AEs in treatment for RA, referred to the last week. Each item has a maximum of 5 questions related to health dimensions, with 4 options of Likert-type responses (from ‘nothing’ to ‘very much’) that assess the impact of this AE on each health dimension (daily activity, social, psychological–cognitive, energy–vitality and general health status). The overall score provides information on the patient's degree of tolerability: the greater the score, the lower the tolerability. The score for each dimension offers information on the impact of each AE on patient's life. These scores were adjusted and transformed from 25 to 100 (from ‘not at all affected’ to ‘very affected’).
Design, Sample Characteristics and VariablesThe validation of the TOL-AR-18 described below was carried out with a cross-sectional, observational, multi-centre study with consecutive inclusion of patients with RA from 6 Rheumatology departments from Barcelona and Balearic Islands area. The patients, who were 18 years of age and older and of both genders, had to have been receiving any treatment for RA at least three months before their inclusion in the study and not to have other diseases that might interfere with the results.
At baseline, socio-demographic variables (age, gender, level of education, professional situation and marital status), clinical variables (date of diagnosis, DAS-28 score, treatment and AEs at the time of the visit) were collected. For each item (AE) of the TOL-AR-18 clinicians should assess the intensity and severity of the event. In addition, patients completed the TOL-AR-18 and the ARthritis Treatment Satisfaction questionnaire (ARTS) and a question about their general health status in relation to RA with 7 options of Likert-type response (from ‘very good’ to ‘very bad’).
The ARTS questionnaire, validated in Spanish population,16 assesses patient satisfaction with treatment by means of 18 items divided into four dimensions, including satisfaction with tolerability dimension (items 9 and 10): ‘I am very concerned about the potential side effects of my medication for osteoarthritis’ and ‘The side effects of my medication for osteoarthritis bother me a great deal’.
Data AnalysisThe Statistical Analysis System (SAS) package for Windows version 9.2 was used for the analysis. In all statistical tests, a significance level P<.05 was used. Regarding psychometric properties of TOL-AR-18, feasibility was analyzed by the evaluation of no-answer percentage of each item. Cronbach's alpha index was used to estimate the reliability of the TOL-AR-18 in terms internal consistency. Also, construct validity was evaluated using Kruskal–Wallis test in order to compare the mean score of 5 subscales and total score between groups of patients according to disease activity (DAS-28) and number of AEs. The hypotheses is that patients with higher activity and more AEs may have higher scores and therefore lower tolerability. We estimated Spearman's correlation coefficient with the ARTS questionnaire score (convergent validity), in the way that a higher tolerability is related to a greater satisfaction. Tol-AR-18 score were compared according to the general health status question.
Roche pharma collaborated with the organization of a first meeting for the researchers and giving statistical support and a small collaboration in the edition of the first draft of the document. The main idea, proposal and management of the study was initiated by the researchers, and the PI edited the full and final document here presented. The researchers do no present any conflict of interest for the elaboration of this document (Table 1).
TOL-AR-18 Questionnaire Itemsa
| TOL-AR-18 questionnaire (Evaluation of the tolerability of treatment for rheumatoid arthritis) |
|---|
| 1. Due to the treatment that I am taking for rheumatoid arthritis, I have more pain in general. |
| 2. Due to the treatment that I am taking for rheumatoid arthritis, I have difficulty in breathing (cough, mucus, feeling of breathlessness). |
| 3. Due to the treatment that I am taking for rheumatoid arthritis, I have local skin reactions in the area of the injection (pain, swelling, bruising). |
| 4. Due to the treatment that I am taking for rheumatoid arthritis, I have fatigue or feel tired. |
| 5. Due to the treatment that I am taking for rheumatoid arthritis, I have general discomfort. |
| 6. Due to the treatment that I am taking for rheumatoid arthritis, I have an increase in blood pressure. |
| 7. Due to the treatment that I am taking for rheumatoid arthritis, I have nausea, heartburn or stomach pains. |
| 8. Due to the treatment that I am taking for rheumatoid arthritis, my hair is falling out more than it used to. |
| 9. Due to the treatment that I am taking for rheumatoid arthritis, I have dizziness and/or vertigo. |
| 10. Due to the treatment that I am taking for rheumatoid arthritis, I have headaches. |
| 11. Due to the treatment that I am taking for rheumatoid arthritis, I have gained weight. |
| 12. Due to the treatment that I am taking for rheumatoid arthritis, I have lost weight. |
| 13. Due to the treatment that I am taking for rheumatoid arthritis, I have palpitations (my heart beats faster than usual). |
| 14. Due to the treatment that I am taking for rheumatoid arthritis, I have infections or changes (skin, respiratory, urine, etc.). |
| 15. Due to the treatment that I am taking for rheumatoid arthritis, I have problems with my mouth (dryness, ulcers, etc.). |
| 16. Due to the treatment that I am taking for rheumatoid arthritis, I have problems with my vision. |
| 17. Due to the treatment that I am taking for rheumatoid arthritis, I have problems with my sex life (reduction in libido, genitourinary problems, etc.). |
| 18. Due to the treatment that I am taking for rheumatoid arthritis, I have sleep problems (insomnia, somnolence). |
The study sample included 66 patients with socio-demographics and clinical characteristics as described in Table 2. AEs reported by patients in the TOL-AR-18 were assessed by clinicians like mild/moderate and not serious. Patients showed less tolerability, meaning higher scores facing all AEs compared with the clinician's assessment in all dimensions (P<.05) as seen in Table 3. The most common event present were hair loss and tiredness/fatigue (Table 4). Pain, tiredness and general discomfort caused by treatment, affected more than 15% of patients very much or quite a lot (Table 5). Clinicians have to ask for other possible AEs, but no AEs apart from those included in the questionnaire occurred at the time of the study. Regarding tolerability, patients had an overall mean (SD) score on the TOL-AR-18 of 29.8 (7.0). The dimensions a little more affected were ‘general health’ and ‘psychological–cognitive’, although all dimensions had very low mean scores (Table 6).
Socio-demographics and Clinical Description of Sample Population.
| Characteristics of the sample | Mean (SD) | % (n) |
|---|---|---|
| Age, years | 56.7 (10.5) | |
| Gender, female | 66.7 (44) | |
| Level of education, primary | 36.4 (24) | |
| Work situation, retired | 47.0 (31) | |
| Civil status, living in couple | 65.2 (43) | |
| Disease duration, years | 10.8 (8.2) | |
| DAS-28 | ||
| Remission/low activity (DAS-28≤3.2) | 65.1 (43) | |
| Moderate/high activity (DAS-28>3.2) | 34.9 (23) | |
| Treatment for RA at the time of the studya | ||
| Synthetic DMARD | 75.8 (50) | |
| Biological DMARD | 42.4 (28) | |
| Corticosteroids | 37.9 (25) | |
| NSAIDs | 34.8 (23) | |
| Other | 9 (6) | |
Patients Report of AEs Intensity, Its Impact in Social/health Dimension Atributted to TOL-AR-18 Scores.
| Dimension | Mild | Moderate | P-value |
|---|---|---|---|
| Daily life | |||
| Mean (SD) | 2967 (5.89) | 3814 (1102) | .0116 |
| N valid | 22 | 13 | |
| Social dimension | |||
| Mean (SD) | 2705 (2.76) | 3553 (9.47) | .0040 |
| N valid | 21 | 12 | |
| Psychological/cognitive dimension | |||
| Mean (SD) | 2998 (7.94) | 4067 (1101) | .0003 |
| N valid | 24 | 14 | |
| Energy/vitality | |||
| Mean (SD) | 2904 (7.31) | 3807 (9.89) | .0031 |
| N valid | 24 | 11 | |
| General health status | |||
| Mean (SD) | 3144 (1076) | 4156 (1296) | .0009 |
| N valid | 25 | 13 | |
| Overall score | |||
| Mean (SD) | 2944 (6.03) | 3729 (8.24) | .0010 |
| N valid | 25 | 13 | |
Percentage of AEs Reported by Patients Included in the TOL-AR-18 Questionnaire.
| AE included in TOL-AR-18 | % (n=60) |
|---|---|
| Hair loss | 27.3 (18) |
| Tiredness/fatigue | 25.8 (17) |
| Weight gain | 24.2 (16) |
| Dizziness and/or vertigo | 22.7 (15) |
| Mouth problems | 22.7 (15) |
| Infection/changes | 21.2 (14) |
| Nausea/heartburn/stomach pain | 19.7 (13) |
| Local skin reactions (injection zone) | 18.2 (12) |
| General discomfort | 18.2 (12) |
| Problems with sex life (reduction in libido, genitourinary problems) | 18.2 (12) |
| Increase in blood pressure | 15.2 (10) |
| Sleep problems | 15.2 (10) |
| Palpitations | 13.6 (9) |
| Problems with vision | 13.6 (9) |
| Difficulty in breathing | 10.6 (7) |
| Headaches | 9.1 (6) |
| More pain in general | 9.1 (6) |
| Weight loss | 1.5 (1) |
Impact of AEs Requested and Included in the TOL-AR-18 Questionnaire.
| AE included in TOL-AR-18 | Very much affected (n) % | Highly affected (n) % |
|---|---|---|
| More pain in general | 4 (6.2%) | 10 (15.4%) |
| Difficulty in breathing | 2 (3.1%) | 5 (7.7%) |
| Local skin reactions (injection zone) | 2 (3.1%) | 3 (4.6%) |
| Tiredness/fatigue | 5 (7.7%) | 15 (23.1%) |
| General discomfort | 4 (6.2%) | 11 (16.9%) |
| Increase in blood pressure | 1 (1.5%) | 5 (7.7%) |
| Nausea/heartburn/stomach pain | 1 (1.5%) | 2 (3.1%) |
| Hair loss | 4 (6.2%) | 7 (10.8%) |
| Dizziness and/or vertigo | 3 (4.6%) | 6 (9.2%) |
| Headaches | 1 (1.5%) | 1 (1.5%) |
| Weight gain | 4 (6.2%) | 9 (13.8%) |
| Weight loss | 0 | 0 |
| Palpitations | 2 (3.1%) | 5 (7.7%) |
| Infection/changes | 4 (6.2%) | 7 (10.8%) |
| Health problems | 3 (4.6%) | 9 (13.8%) |
| Problems with vision | 4 (6.2%) | 9 (13.8%) |
| Problems with sex life (reduction in libido, genitourinary problems) | 5 (7.7%) | 9 (13.8%) |
| Sleep problems | 3 (4.6%) | 6 (9.2%) |
Scale from: “patients’ life very much affected” ranging to “highly affected”.
Mean and Impact of the TOL-AR-18 Questionnaire Dimensions in Social/health Variables.
| Dimension | Mean (SD)a |
|---|---|
| Daily life | |
| Mean (SD) | 30.0 (7.9) |
| N valid | 57 |
| Social dimension | |
| Mean (SD) | 28.2 (6.1) |
| N valid | 55 |
| Psychological/cognitive dimension | |
| Mean (SD) | 30.8 (9.3) |
| N valid | 60 |
| Energy/vitality | |
| Mean (SD) | 29.5 (7.8) |
| N valid | 57 |
| General health status | |
| Mean (SD) | 31.7 (10.8) |
| N valid | 60 |
The psychometric properties assessed in the validation of the tolerability questionnaire are described below.
The rate of non-response to the 18 items was 1.5%. Reliability of the TOL-AR-18 questionnaire was assessed in terms of internal consistency with, Cronbach's α values greater than 0.95 for each item, which indicates that all items were very inter-related. Correlation between each item and overall score was greater than 0.3 that is a satisfactory correlation.
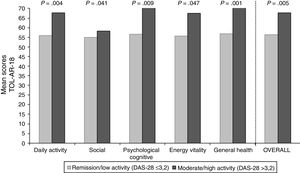
To asses the construct validity, TOL-AR-18 scores were compared with DAS-28 and with number of AEs. Patients with moderate/high activity had higher scores (lower tolerability) (Fig. 1), with statistically significant differences when compare with all other patients (P<.05). This significance was due to patients in remission who have very low scores. Statistically significant differences were also found for patients without AEs compared to all other patients. Patients with ≥2 AEs showed higher scores. To asses the construct validity, also Spearman's correlation coefficient (Rho) between TOL-AR-18 dimensions and ARTS questionnaire scores (items 9, 10 and tolerability dimension) was calculated. The dimensions of the questionnaire TOL-AR-18 had an inverse and low-moderate correlation with ARTS questionnaire (item 9 and item 10). Item 10 of ARTS had higher correlation coefficients with the dimensions of the TOL-AR-18 than item 9: between 0.21 and 0.38 and between 0.09 and 0.34 respectively. ‘General health status’ and ‘psychological/cognitive’ dimensions of TOL-AR-18 showed the highest correlation coefficients with tolerability dimension of the ARTS while ‘energy-vitality’ showed the lowest (Table 7). In addition, in the analysis of answers to the question about general health status, differences in the ‘daily activity’ and ‘psychological–cognitive’ dimensions and overall score for the TOL-AR-18 were found to be statistically significant (P=.008; 0.024; 0.041). Patients who assessed their general health status as ‘somewhat poor’ obtained a higher score (lower tolerability) on this questionnaire.
Correlation Between TOL-AR-18 Dimensions and ARTS Questionnaire (Items 9, 10 and Tolerability Dimension).
| TOL-AR-18 dimensions | ARTS | ||
|---|---|---|---|
| Item 9 | Item 10 | Tolerability dimension | |
| Daily life | Rho=−0.23 P-value=.079 n=57 | Rho=−0.34 P-value=.012 n=55 | Rho=−0.370 P-value=.005 n=57 |
| Social dimension | Rho=−0.25 P-value=.072 n=55 | Rho=−0.25 P-value=.074 n=53 | Rho=−0.291 P-value=.031 n=55 |
| Psychological/cognitive dimension | Rho=−0.27 P-value=.038 n=60 | Rho=−0.40 P-value=.002 n=58 | Rho=−0.426 P-value=.001 n=60 |
| Energy/vitality | Rho=−0.09 P-value=.528 n=57 | Rho=−0.21 P-value=.119 n=55 | Rho=−0.197 P-value=.142 n=57 |
| General health status | Rho=−0.34 P-value=.008 n=60 | Rho=−0.38 P-value=.003 n=58 | Rho=−0.438 P-value<.001 n=60 |
| Total score | Rho=−0.29 P-value=.028 n=59 | Rho=−0.36 P-value=.006 n=57 | Rho=−0.400 P-value=.002 n=59 |
Spearman's correlation coefficients (Rho).
Assessment of tolerability of drug therapy for RA is a largely unexplored condition. Despite this, the treatment compliance rate is directly related to these non-serious AEs, and they are sometimes a reason for treatment withdrawal. In clinical trials, PROs are essential and show the patient's perspective in the conduct of a trial with a new drug. Similarly, in daily clinical practice, including patient's perspective in the management of their disease is on the increase, especially in chronic diseases.4,17,18
We aimed to show in the present study that the TOL-AR-18 questionnaire could present good psychometric properties, although it presents some limitations. It has a very low rate of non-response, so its administration at rheumatology visits is feasible. The high internal consistency of its items, greatly exceeding the expected theoretical values,19 is an excellent indicator of their homogeneity, all forming part of the same construct: tolerability. On the other hand, the questionnaire's low scores are consistent with the profile of the population studied, with low disease activity and little presence of AEs, it is indicating a good construct validity. In addition, we also observed a trend towards having lower tolerability (higher score) in patients with more active disease, but this is not statistically significant. This means that the TOL-AR-18's discriminant capacity would probably be confirmed were the questionnaire to be administered to a more heterogeneous population in terms of disease activity and presence of AEs. This hypothesis would need to be confirmed with further studies. Finally, correlations with the ARTS questionnaire were moderate–low, given that this questionnaire (ARTS) assesses treatment satisfaction, not directly tolerability as TOL-AR-18 does. Although the tolerability dimension (items 9 and 10 of the ARTS) theoretically assesses this concept, the TOL-AR-18 questionnaire assesses specific AEs, whereas the ARTS asks in general about side effects of treatment and maximum degree of difficulty. This degree of trouble was not very present in the study patients, as shown by the low percentage of patients who assessed the degree of difficulty due to an AE with the maximum score. Specifically, in relation to adverse events reported, after the study we were unable to obtain more powerful conclusions. Likewise, the information contained in the questionnaire was 100% reflected by the patients and that it coincides mostly with those selected by the rheumatologists. Therefore, the TOL-AR-18 is more specific for assessing tolerability.
Patient involvement in the management of their disease is becoming increasing important, as is having their self-assessment of various aspects of health available. Traditionally, tolerability in any disease is assessed from the clinical perspective of the health personnel in taking care of patient management and follow-up, by means of laboratory tests or by lists of AEs.20,21 It is also obtained by asking the patient directly. However, the patient may be asked very general questions or questions aimed at AEs that are more typical or serious or that can be expected depending on treatment regimen. Hence, the patient may not mention a very mild symptom because he/she does not believe it to be important, because he/she is not asked about it directly or because he/she believes it is normal and therefore does not report it. This study were conducted to obtain a useful, simple-to-use and reproducible tool and to validate its utility in daily clinical practice.
As a result of the need to obtain information about tolerability from the patient's perspective that may not have been obtained in the course of clinical assessment, questionnaires have been designed and validated in areas such as psychiatry, ophthalmology and gastroenterology9,22,23; but to date no questionnaires have been launched to assess the impact of AEs in the area of Rheumatology, specifically in RA.
We believe that, with the TOL-AR 18, an easy-to-use, standardized examination tool, with good measurement properties that can be self-administered by patients, available for professionals who care for RA patients on a daily basis, we answer the need that currently exists. It complements these professionals’ medical knowledge without overloading a medical visit. This questionnaire not only quantifies treatment-related symptoms, but also is valid for detecting potential adverse reactions not revealed with other kind of assessment. It also offers the novelty of quantifying the degree of trouble or importance for the patient of a particular AE, which could be greater than previously believed by the clinician. Up to now, this feature of the TOL-AR-18 might not have been gathered during visits, since it has to do with a more subjective aspect. Given that the TOL-AR-18 allows any health professional to perform a quick, detailed examination of AEs, it may be useful not only for rheumatologists, but also nursing staff and in general all professionals involved in some way in follow-up of a patient with RA.
The information obtained with this questionnaire will provide additional information about the different therapeutic strategies available and will help to optimize management of the patient with RA in terms of decision-making. Decisions might concern changes in treatment, dose or even route of administration. Thus, the information obtained will improve the patient's satisfaction with his/her treatment and HRQoL and involve him/her in management of the disease, and as a result the physician–patient relationship will be strengthened. Our results suggest a potential extrapolation to other types of rheumatic and joint diseases with therapeutic arsenals similar to those of RA. However, this will require the relevant validations of the TOL-AR-18 questionnaire in other patient populations.
This study did have some limitations. First and foremost, we consider that using DAS28 index and the number of AEs to asses the construct validity of TOL-AR-18 could be complicated, because disease activity may influence the perception of side effects by the patient. Maybe patients with high disease activity may tolerate better minor side effects than those with lower disease activity since they are more dependent of the beneficial effect of the treatment. Further studies to asses this issue may help to better understand the behaviour of the patients. Other limitations of particular note are the following: (1) Some limitations were detected in the questionnaire validation phase, such as a limited patient sample size (66 patients). Furthermore, owing to the effect of chance (consecutive enrolment), the sample turned out to be rather homogeneous with respect to disease activity and presence of AEs. Some of the questionnaire's measurement properties could probably have been assessed better with a larger, more heterogeneous sample. (2) The limitations inherent to a cross-sectional study hindered assessment of properties such as test–retest reliability, i.e. reproducibility of responses in patients with stable or similar clinical conditions or sensitivity to change that could have allowed changes in TOL-AR-18 scores to be assessed during a period of time in comparison with clinical variables. 3) The fact that a satisfaction questionnaire for patients with osteoarthritis such as the ARTS was chosen to analyze its correlation with the TOL-AR-18 might initially seem questionable. It should be noted that this questionnaire was originally designed and validated in a population with RA24 although in the Spanish validation it was used in patients with osteoarthritis. How there are not questionnaires about tolerability validated in Spanish and ARTS has a specific domain (two items) for tolerability, we chose this questionnaire instead of a generic questionnaire. Despite this, it was decided to use it to assess the effects of treatments for rheumatic and joint diseases, we believed to be more appropriate than a generic or HRQoL questionnaire for RA as it had a tolerability dimension.
To summarize, the TOL-AR-18 questionnaire is hereby presented as a support tool for professionals involved in the management of RA. It offers them a standardized means of exploring and detecting potential AEs and furthermore strengthens the physician–patient relationship by involving the patient in the disease follow-up and outcome. We therefore, due to the limitations of our study suggest, that TOL-AR-18 is a complementary tool in the management and evaluation of tolerability in RA. We suggest that the use of the questionnaire in daily clinical practice could ease a more fluent and patient-oriented management, leading to optimal control of patients with chronic arthritis.
Conflict of InterestNone for the topic of the project.
The authors would like to thank Liliana Ercole from Roche Frama, S.A. for her contribution providing support to carry out this project.
The authors would also give thanks to the Fundació Parc Taulí from Sabadell, for their support as sponsor of the study.