To study the prevalence of liver fibrosis (LF) measured by FibroScan and APRI index in patients with rheumatoid arthritis (AR) undergoing treatment with methotrexate (MTX).
MethodsWe included 59 patients with RA on MTX. Medical records, FibroScan measures and serological markers of liver damage were compared on the basis of cumulative methotrexate dose.
ResultsMean treatment duration was 82.4±65.1 months and mean cumulative dose was 5214.5±4031.9mg. Five patients met LF criteria by fibroscan, while only one patient had a suggestive APRI score. No statistically significant differences were found in terms of LF measured by both APRI and fibroScan between patients with cumulative doses above and below 4000mg. There was also no relationship between LF and treatment duration.
ConclusionsThe occurrence of LF in patients with RA on MTX is a multifactorial process that does not seem directly related to its cumulative dose. FibroScan may be a useful technique in clinical practice to screen for this complication.
Estudiar la prevalencia de la fibrosis hepática (FH) medida por FibroScan e índice APRI en pacientes con artritis reumatoide (AR) en tratamiento con metotrexato (MTX).
MétodosSe incluyeron 59 pacientes con AR en tratamiento con MTX. Se compararon las historias clínicas, las mediciones de FibroScan y los marcadores serológicos de daño hepático en función de la dosis acumulada de MTX.
ResultadosLa duración media del tratamiento fue de 82,4±65,1 meses y la dosis media acumulada de 5214,5±4031,9mg. Cinco pacientes cumplían criterios de FH por FibroScan y un solo paciente por APRI. No se encontraron diferencias estadísticamente significativas en cuanto a FH tanto por APRI como por FibroScan en base a dosis acumuladas superiores o inferiores a 4000mg. Tampoco hubo relación entre FH y duración del tratamiento.
ConclusionesLa FH en pacientes con AR tratados con MTX es un proceso multifactorial sin aparente relación directa con la dosis acumulada. El FibroScan puede ser una técnica útil en la práctica clínica para detectar esta complicación.
Methotrexate (MTX) is a folic acid analogue that inhibits the synthesis of purines and pyrimidines. It is widely used in the treatment of chronic inflammatory diseases such as rheumatoid arthritis (RA). MTX is usually the first drug of choice in the treatment of RA.
Adverse events related to MTX hepatotoxicity include increased transaminases and liver fibrosis (LF). Thus, international guidelines recommend transaminase monitoring every 1–3 months.1,2
Liver biopsy is the gold standard for the diagnosis of LF,3 and it is currently the technique of choice when studying persistent abnormalities in liver function in patients treated with MTX.4 However, it has important limitations that do not allow its use as a screening technique. Firstly, it is an invasive technique that is not risk-free and, in addition, there may be a disparity of values in different samples.5 Thus, the non-invasive study of LF using serum markers, radiological studies and transitional elastography is now being proposed.
Transient elastography using FibroScan (FS) makes it possible to study a volume 100 times larger than that of a biopsy sample, and is therefore more representative of the liver parenchyma.6 Moreover, since it is a non-invasive, rapid and easily reproducible technique (inter- and intra-observer correlation of 98%7) it allows serial measurements in order to study disease progression. This technique is already available for LF screening in other conditions such as viral hepatitis.8 In the case of RA patients on MTX, Olsson-White et al.9 determined for FS a sensitivity of 100% and a specificity of 84% in detecting LF by comparing fibroscan with APRI, FIB4 and hepascore.
On the other hand, LF can present with transaminase levels in range. Therefore, screening by laboratory studies requires combined indices such as the APRI index resulting from the ratio of aminoaspartate transaminase (AST) and platelet levels (APRI). The usefulness of APRI score for identifying liver fibrosis has been extensively studied in HIV/HCV. Compared to biopsy with a sensitivity at the 0.7 threshold of 77% and specificity of 72% for the detection of significant LF.10
ObjectivesThe aim of our study was to assess the prevalence of LF measured by FS and APRI index in patients with RA treated with MTX in our hospital. In addition, we studied its relationship with treatment duration (TD) and cumulative dose of MTX (CD).
MethodsPatients were recruited between the 1st February 2019 and the 31st January 2020 from the rheumatology clinics of a single centre (Hospital Universitario Donostia). A total of 59 patients aged 18 years or older were prospectively studied. All had been diagnosed with RA by a rheumatologist and on treatment with MTX (without limitation in the duration of treatment).
Demographic and analytical data, treatment history and CD were collected by review of the computerized medical records. LF was defined by FS (measurement greater than 7Kpa) and by APRI score (result greater than 0.7). The FS assessment was performed by a trained nurse using the Fibroscan®402 (Echosens, Paris, France). FS is an ultrasound-based technique that allows estimation of liver stiffness based on the transmission speed of shear waves.11 At the time of inclusion in the study a blood test was performed to calculate APRI scores. High transaminase levels were defined as results above 33U/L. Finally, disease activity was defined by a rheumatologist using the DAS28-CRP score (a score below 0.6 equals remission, between 0.6 and 3.2 equals low activity, between 3.2 and 5.1 moderate activity and scores above 5.1 equals high activity).
Exclusion criteria were prior diagnosis of liver disease (hepatitis B or C virus infection; known non-alcoholic fatty liver disease), alcohol consumption greater than 60g/day in men or 40g/day in women, HIV infection on antiretroviral therapy, diabetes mellitus, chronic renal failure, congestive heart failure or body mass index (BMI) greater than 30kg/m2. Patients receiving leflunomide in the 3 years prior to the study were also excluded. The study was approved by the clinical research ethics committee of the Gipuzkoa health area and participants signed an informed consent form prior to inclusion. Data were collected by means of a questionnaire, a review of the computerized clinical history and a visit.
The primary outcomes of the study were, first, the percentage of patients with LF measured by FS or APRI in our sample. Secondly, to study the relationship between LF and cumulative MTX dose or treatment duration. The secondary outcomes studied were the relationship with liver enzymes and metabolic syndrome (MetS).
Statistical analysisWe initially performed a descriptive analysis by calculating the mean and standard deviation (or median and interquartile range) for quantitative variables. For qualitative variables, we calculated the absolute and relative frequencies in percentages. We performed the Chi-square test or Fisher's test to compare the distribution of qualitative variables. Similarly, we used Student's t-test or Mann–Whitney U-test to compare quantitative variables. All analyses were performed with STATA 16.1.
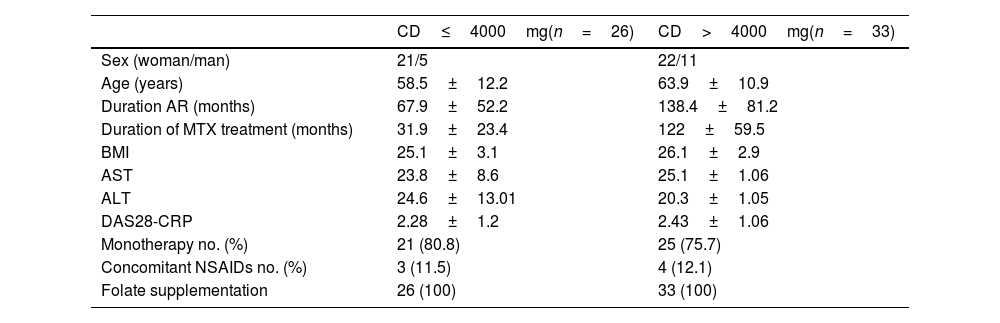
OutcomesInitial dataFifty-nine patients were included with a mean treatment duration (TD) of 82.4 months (standard deviation, SD, 65.1). The mean CD was 5214.5mg (SD 4031.9): 26 patients had an CD less than or equal to 4000mg and 33 had a CD greater than 4000mg. Demographic variables are summarized in Table 1. TD and disease progression time were longer in the group with a TD higher than 4000mg. Forty-six patients (77.9%) were on MTX monotherapy, and only 7 (11.8%) were taking NSAIDs.
Baseline characteristics.
| CD≤4000mg(n=26) | CD>4000mg(n=33) | |
|---|---|---|
| Sex (woman/man) | 21/5 | 22/11 |
| Age (years) | 58.5±12.2 | 63.9±10.9 |
| Duration AR (months) | 67.9±52.2 | 138.4±81.2 |
| Duration of MTX treatment (months) | 31.9±23.4 | 122±59.5 |
| BMI | 25.1±3.1 | 26.1±2.9 |
| AST | 23.8±8.6 | 25.1±1.06 |
| ALT | 24.6±13.01 | 20.3±1.05 |
| DAS28-CRP | 2.28±1.2 | 2.43±1.06 |
| Monotherapy no. (%) | 21 (80.8) | 25 (75.7) |
| Concomitant NSAIDs no. (%) | 3 (11.5) | 4 (12.1) |
| Folate supplementation | 26 (100) | 33 (100) |
CD: cumulative dose of methotrexate; SD: standard deviation; BMI: body mass index.
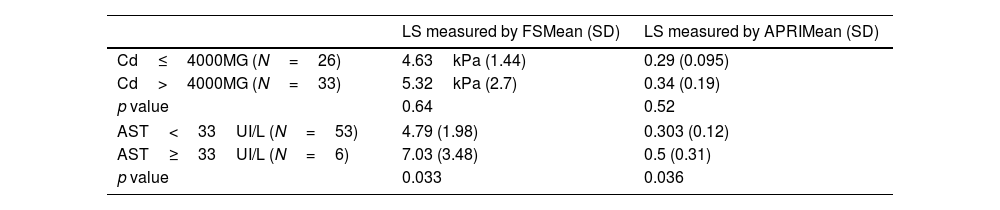
Five patients (8.5%) had liver stiffness above 7Kpa suggestive of LF. In contrast, only one patient fulfilled criteria for LF by APRI score.
The mean FS value was 4.63kPa±1.44 in the group with CD less than or equal to 4000mg and 5.32kPa±2.7 in the group with higher CD. No statistically significant differences were found in terms of LF measured by both APRI (p=0.52) and FS (p=0.64) between patients with CD above and below 4000mg (Table 2). No association was found between LH and TD, either measured by FS (p=0.66) or by APRI (p=0.61).
Relationship between CD/AST and liver stiffness measured by FS and APRI.
| LS measured by FSMean (SD) | LS measured by APRIMean (SD) | |
|---|---|---|
| Cd≤4000MG (N=26) | 4.63kPa (1.44) | 0.29 (0.095) |
| Cd>4000MG (N=33) | 5.32kPa (2.7) | 0.34 (0.19) |
| p value | 0.64 | 0.52 |
| AST<33UI/L (N=53) | 4.79 (1.98) | 0.303 (0.12) |
| AST≥33UI/L (N=6) | 7.03 (3.48) | 0.5 (0.31) |
| p value | 0.033 | 0.036 |
CD: cumulative methotrexate dose; LS: liver stiffness; LF: liver fibrosis; FS: fibroscan; SD: standard deviation.
Within the included patients, 9 (15.25%) had MetS. No significant differences were found in APRI or FS between patients with MetS and the rest of the sample.
Regarding transaminase levels (Table 2), an association was found between AST higher than 33IU/L and higher values in FS (p=0.033) and APRI (p=0.036). In the case of ALT, a relationship was only found between elevated levels and APRI (p=0.0005).
Discussion and conclusionsRA is the most common chronic inflammatory arthritis in Spain, affecting 0.82% (95% CI: 0.59–1.15) of the adult population.12 Despite the emergence of new therapeutic targets in recent years, MTX remains the cornerstone of RA treatment, both in monotherapy and in association with biological therapies.1,13
Although chronic MTX use has classically been associated with liver fibrosis and even cirrhosis, there is growing evidence that these complications are largely affected by other factors such as alcoholism, concomitant use of other drugs or the presence of MetS.14–17
In our sample, only 8.5% of patients had liver stiffness above 7.0kPa. Similarly, the remaining studies published to date evaluating the association between MTX and LF assessed by FS have shown low prevalence of LF (6–12%). Of the reviewed studies, only two confirmed the diagnosis histologically by biopsy. Thus, Arena et al.18 performed liver biopsy in 5 of the 11 patients with FS>7kPa. Of these, 3 had no evidence of fibrosis but minimal signs of lobular inflammation. Lahari et al.19 performed a histological study in 10 patients treated with MTX, of whom only one had a diagnosis of RA. This case showed evidence of non-alcoholic hepatic steatosis, but no signs of fibrosis.
Like us, others have previously found no significant association between CD or TD of MTX and LF measured by FS. Thus, Park et al.6 in a prospective study of 177 patients, found no significant differences in FS measurements based on CD above or below 4000mg or with respect to healthy controls. Lahari et al.19 conducted a study of 390 patients with chronic inflammatory diseases, 149 of whom were diagnosed with RA with a minimum CD of 1500mg. In this case, they also found no correlation between LF and MTX use in comparison with controls, nor depending on CD. Similar results were obtained by Barbero-Villares et al.5 in a prospective study of 53 patients on chronic MTX treatment. In this study 17 patients diagnosed with RA were included. They had a mean CD of MTX of 2635mg. Although the CD was higher than for the other conditions (psoriasis and inflammatory bowel disease), there were no significant differences in FS outcomes across the groups. Furthermore, Feuchtenberger et al.20 compared the presence of LF in RA patients on MTX versus patients without MTX exposure (n=119), also finding no differences between both groups. Finally, Kumar et al.21 had similar results with a sample of 63 patients and a mean CD of 4225mg.
In contrast, Arena et al.,18 in their 100-patient study with a minimum CD of 1500mg, did observe a significant association between CD (above or below 4000mg) and liver stiffness. Bafna et al.,22 with 75 patients, had similar results while also finding that patients with LF had a significantly larger abdominal circumference. In the same line, Lertnawapan et al.23 described a higher CD in patients with LF, but also a significantly higher proportion of hyperlipaemia, hepatic steatosis and higher BMI.
Regarding the LF by APRI, our study does not allow conclusions as only one patient had a score higher than 0.7. Previous studies have also shown no relationship between APRI and CD.6,23 In parallel correlation between APRI score and FS appears to be good in RA patients treated with MTX.6 In contrast, Olson-White et al. found a lower sensitivity and specificity for LF detection by APRI than FS.9
As for the relationship of liver stiffness measurements by FS and transaminases, in our study we found significant differences in terms of AST but not ALT. In this direction, Arena et al.18 described in their sample a weak relationship between FS and ALT measurements while Lertnawapan et al.23 showed significantly higher levels of ALT, AST and GGT in patients with LF. In the case of Lertnawapan et al., as mentioned above, patients with LF had a significantly higher proportion of other risk factors for liver damage. The rest of the studies analyzed showed no association between liver stiffness measured by FS and transaminase levels. In parallel, studies comparing the use of combined scores6,19,20,23 with FS, as in our case, found no correlation between them. This supports the idea that LF may develop with transaminases in the normal range, making it necessary to search for new screening techniques independent of transaminases.
On the other hand, obesity is a factor that could act to increase susceptibility to MTX-induced liver damage, aggravating pre-existing non-alcoholic fatty liver disease.24,25 This may explain the results of some of the studies reported.22,23 Another factor that could have an effect on the development of fibrosis in our patients is adequate folic acid supplementation. A review of 47 studies26 described a cumulative incidence of LF per biopsy of more than 15%, with folate supplementation of 9%. The same review associated the presence of LF with high NSAID consumption (67%). In our sample, folate supplementation was of 100% and 37.3% of patients consumed NSAIDs. Finally, persistent inflammation in RA itself is a factor to be taken into account in the development of liver disease.27,28
In conclusion, the presence of LF in RA patients treated with MTX is a rare event. The development of LF is a multifactorial process in which MTX does not act in isolation, so it is not directly related to the CD of MTX. In contrast, MTX may aggravate liver damage in patients with other associated risk factors. Thus, we do not consider that the presence of LF requires discontinuation of MTX, although it does require closer monitoring of the patient.
The results of both our study and previous bibliography suggest that screening for LF based solely on periodic transaminase measurements may be insufficient. In this context, the use of FS seems useful in selected patients. However, further studies are still needed to define in which patients and at what point in time this test would be indicated.
Consents and approval of the ethics committeeThe publication of the case was approved by the ethics committee of the Donostia University Hospital. Informed consent was obtained from the patient for the publication of his case.
Conflicts of interestThe authors declare they have no conflicts of interest.
Andrea de Diego-Sola (Department of Rheumatology, Hospital
Universitario Donostia, San Sebastián, Spain)
Agustin Castiella Eguzkiza (Department of Gastroenterology,
Hospital Universitario Donostia, San Sebastián, Spain)
Luis María López Domínguez (Department of Rheumatology,
Hospital Universitario Donostia, San Sebastián, Spain)
Iratxe Urreta Barallobre (Department of Clinical Epidemiology,
Hospital Universitario Donostia, San Sebastián, Spain)
María José Sánchez Iturri (Department of Gastroenterology,
Hospital Universitario Donostia, San Sebastián, Spain)
Cesar Antonio Egües Dubuc (Department of Rheumatology, Hospital Universitario Donostia, San Sebastián, Spain)
Jorge Jesús Cancio Fanlo (Department of Rheumatology, Hospital
Universitario Donostia, San Sebastián, Spain)
Olga Maíz Alonso (Department of Rheumatology, Hospital Universitario Donostia, San Sebastián, Spain)
Jesús Alejandro Valero Jaimes (Department of Rheumatology,
Hospital Universitario Donostia, San Sebastián, Spain)
María Vaamonde Lorenzo (Department of Gastroenterology,
Hospital Universitario Donostia, San Sebastián, Spain)
Leire Samaniego Leoz (Department of Gastroenterology, Hospital Universitario Donostia, San Sebastián, Spain)
Joaquín Belzunegui Otãno (Department of Rheumatology, Hospital Universitario Donostia, San Sebastián, Spain)
Eva María Zapata Morcillo (Department of Gastroenterology,
Hospital Universitario Donostia, San Sebastián, Spain)