Serum vascular endothelial growth factor (VEGF) levels correlate with structural alterations in Rheumatoid Arthritis (RA). Since P wave dispersion (PWD) is associated with atrial ischemic-related fibrotic changes, it was conceived that there may be a correlation between altered PWD and increased VEGF levels in RA.
MethodsIn this prospective observational study, we evaluated patients with RA, and compared them to control subjects. PWD was considered as the difference between the maximum and minimum duration of the P wave. An altered PWD was considered one that had dispersion≥38ms. Measurements of VEGF serum levels were performed using enzyme-ligand, immunosorbent measurement ELISA kits.
ResultsA total of 99 patients with RA, and 48 control subjects were evaluated. The PWD was 25.3±4.9ms in the control group vs. 57±14.9ms (p<0.0001) in the RA group. No patient in the control group had altered PWD, while 94 (95%) patients in the RA group presented it (p<0.0001). The value of VEGF in the control group was 15.2±15.1pg/ml vs 51.1±55.5pg/ml (p<0.001) in RA. The value of VEGF in RA without altered PWD was 20±12pg/ml vs 56±57pg/ml in RA with altered PWD (p<0.02). An elevated VEGF value had a specificity of 80%, and a positive predictive accuracy of 95% in predicting altered PWD in RA.
ConclusionsThis study establishes for the first time that RA patients who possess significantly higher serum levels of VEGF have an altered PWD. The presence of an elevated VEGF serum value has a high specificity, and high positive predictive accuracy of the existence of altered PWD in RA.
Los niveles séricos del factor de crecimiento endotelial vascular (VEGF) se correlacionan con alteraciones estructurales en la artritis reumatoide (AR). Dado que la dispersión de la onda P (PWD) se asocia con cambios fibróticos relacionados con la isquemia auricular, se concibió que puede haber una correlación entre la PWD alterada y el aumento de los niveles de VEGF en la AR.
MétodosEn este estudio observacional prospectivo, evaluamos a pacientes con AR y los comparamos con sujetos de control. Se consideró la PWD como la diferencia entre la duración máxima y mínima de la onda P. Se consideró la PWD alterada aquella que tenía una dispersión ≥ 38ms. Las mediciones de los niveles séricos de VEGF se realizaron utilizando kits ELISA de medición inmunoabsorvente de enzima-ligando.
ResultadosSe evaluaron un total de 99 pacientes con AR y 48 sujetos controles. La PWD fue de 25,3±4,9ms en el grupo de control frente a 57±14,9ms (P <0,0001) en el grupo de AR. Ningún paciente del grupo control presentó PWD alterada, mientras que 94 (95%) pacientes del grupo AR la presentaron (P<0,0001). El valor de VEGF en el grupo control fue de 15,2±15,1 pg/ml frente a 51,1±55,5 pg/ml en la AR (P <0,001). El valor de VEGF en la AR sin PWD alterada fue de 20±12 pg/ml frente a 56±57 pg/ml en la AR con PWD alterada (P <0,02). Un valor elevado de VEGF tuvo una especificidad del 80% y un valor predictivo positivo del 95% para predecir la PWD alterada en la AR.
ConclusionesEste estudio establece por primera vez que los pacientes con AR que poseen niveles séricos significativamente más altos de VEGF tienen una PWD alterada. La presencia de un valor sérico elevado de VEGF tiene una alta especificidad y un alto valor predictivo positivo de la existencia de PWD alterada en la AR.
Rheumatoid arthritis (RA) is a chronic inflammatory disease of an autoimmune nature that may develop systemic compromise frequently affecting the cardiovascular system.1–3 Cardiac involvement in RA can manifest itself in various ways, and may be asymptomatic, or have mild to severe clinical manifestations with increased clinical morbidity and mortality.1–5 Conduction system abnormalities and cardiac arrhythmias have been observed in RA patients and are clearly associated with premature atherosclerosis and ischemic heart disease in those younger ones.4–8 Various histological and electrophysiological changes in the atrial myocardium, such as fibro-degenerative changes, increased dispersion of refractory periods, delayed impulse conduction, anisotropic conduction, are alterations associated with atrial myocardial vulnerability.9–12 The myocardial fibers obtained from patients with atrial pathology have a high membrane potential, a depression of the maximum amplitude of the action potential and a decrease in the diastolic depolarization velocity that produce a propitious terrain to develop the mechanisms of reentry and induction of atrial arrhythmias.13 These atrial abnormalities may be detected at the electrocardiographic level by an increase in the amplitude, morphology, duration, and dispersion of the P wave, and may be related to the development of atrial arrhythmias.14–17 We have previously shown that these changes in the P wave are related to the recording of abnormally prolonged and fractionated atrial endocardial electrograms by catheter atrial mapping during sinus rhythm.18,19
On the other hand, a significant increase in serum vascular endothelial growth factor (VEGF) related to inflammation has been described in RA patients developing cardiovascular complications in the clinical course of their disease.20–22 Patients with RA may present subtle changes at the level of the atrial myocardium that are detectable with the electrocardiogram, in addition to changes in certain serum biomarkers. The current research was therefore undertaken in RA patients to address the following hypotheses, since P wave dispersion is associated with ischemic-related fibrotic changes and inflammatory alterations in the atrial myocardium,14–17 there may be a correlation between altered P wave dispersion and increased levels of VEGF in patients with RA. Accordingly, since this correlation is not reported yet in the literature, it was prospectively studied in our RA patients.
MethodsStudy patientsPatients 18 years of age or older, of both sexes, diagnosed with RA, were the focus of this prospective, observational, analytical, cross-sectional research. The study patients had their RA diagnosis established by a rheumatologist according to the 2010 Rheumatoid Arthritis classification criteria from the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR).23 The control group consisted of subjects without autoimmune rheumatic disease.
Inclusion criteria in the control group- •
Healthy subjects of both sexes and 18 years of age or older.
- •
Subjects with cardiovascular risks factors, but without decompensated organic heart disease.
- •
The subjects had to be in sinus rhythm with eligible and discernible P waves in the ECG.
- •
Subjects were excluded from the control group if they had autoimmune rheumatic disease.
- •
Subjects with atrial flutter or fibrillation.
- •
Subjects with infectious diseases.
- •
Subjects with electrolyte imbalances.
- •
Subjects with decompensated organic heart disease.
- •
Patients who met the RA established diagnosing criteria were consecutively enrolled.
- •
Patients of both sexes, and 18 years of age or older.
- •
Patients had to be in sinus rhythm with eligible and discernible P waves in the ECG.
- •
Patients had to be enrolled and followed-up with complete medical records in the Rheumatology Department of our Institution.
- •
Patients who complied with all the research steps to be carried out in the study: Collection of clinical data, taking blood samples for analysis, and 12 channel ECG recordings.
- •
Patients who signed the informed consent.
- •
Patients with other autoimmune rheumatic diseases other than RA.
- •
Patients with overlapping diagnosis.
- •
Patients in atrial flutter or fibrillation.
- •
Patients with any kind of infectious diseases.
- •
Patients with electrolyte imbalances.
- •
Patients with chronic obstructive pulmonary disease.
- •
Patients with irregular follow-up.
- •
Patients who do not comply with all the stages of the research.
- •
Patients who refused to participate in the project.
The studies were conducted in accordance with the ethical standards of the WMA Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subject and its later amendments, with the approval of the local institutional ethics committee review board. Informed consent was obtained from all the individuals participating in this research. The work instrument was the Clinical Data Repository in which the clinical, demographics, related laboratory tests and other study variables of interest for this research were recorded on an Excel file format.
Electrocardiographic measurementsThe twelve electrocardiographic leads were recorded at a paper speed of 50mm/s and a voltage of 20mm/mV with a 12-channel electrocardiograph, Cardiofax, Model ECG-9130K, Nihon Kohden Corporation, Tokyo, Japan. Measurements of the P wave in the electrocardiogram were performed in the twelve electrocardiographic leads. The maximum and minimum duration of the P wave was obtained by manual calculation under a magnified view with a loupe in each patient. Two researchers performed the P-wave measurements from the beginning of the first P-wave deflection deviating from the isoelectric line to the end of the P-wave deflection returning to the isoelectric line. The dispersion of the P wave was considered as the difference between the maximum and minimum duration of the P wave, and was measured in milliseconds. An altered or pathological P wave dispersion was considered one that had dispersion≥38ms.16,24
Blood sample collection and immune assayBlood samples were obtained after a 12-h fast and measurements of serum levels of VEGF were performed using enzyme-ligand immunosorbent, the Quantikine human VEGF Immunoassay ELISA kit (R & D Systems INC, USA). EDTA serum specimens were collected from a peripheral vein in a 6ml Vacuette tube containing gel separator and clot activator. No patient had an infectious disease, or sore throat, or flu-like symptoms at the moment of blood sample extractions. This assay employs the quantitative sandwich enzyme immunoassay technique. A monoclonal antibody specific for human VEGF has been pre-coated onto a microplate. Standards and samples were pipetted into the wells and any VEGF present is bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for human VEGF was added to the wells. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution was added to the wells and color develops in proportion to the amount of VEGF bound in the initial step. The color development is stopped and the intensity of the color is measured. Normal VEGF values were considered in the range of 0.15–75.2pg/ml.
Statistical analysisData processing and statistical analyzes were performed in Python 3.7.12. The comparison of the categorical variables between the groups was made using the Fisher exact test. In order to compare quantitative variables between groups a non-parametric test (Mann–Whitney U test) was performed. Linear regressions were performed to demonstrate correlation between the ECG parameters and laboratory blood samples using the Fisher test. Adequate adjustment for confounding factors such as age, gender, disease activity, or medications, were performed through multivariate analysis. The electrocardiograms were reviewed independently by two researchers (JT and CM), and the measurements were entered in duplicate to eliminate interobserver variability. If there was discrepancy between the two measurements, the original electrocardiogram was retrieved and reassessed by the two researchers and reviewed with a third cardiologist (OC), together until a consensus was reached. Kappa values were utilized to determine interobserver variability and reliability for categorical variables; values of 0.81–1.0 are indicative of excellent agreement; 0.61–0.80, substantial agreement; 0.41–0.60, moderate agreement; 0.21–0.40, fair agreement; 0–0.20, slight agreement; and values≤0, poor agreement.25 This method produced an excellent correlation between the two observations with a kappa statistic of 0.90. The sample size was estimated using the Epi info 7.2 software, using a confidence level of 95%, a power of 80%, with an expected frequency of 2%, which yielded a minimum sample size of 82 subjects. Using receiver operating characteristics (ROC) curve, the area under de curve (AUC), for diagnostic value and accuracy of different studied parameters were calculated utilizing the Epidat 3.123 program software package. Specifically, the ROC curve of the presence of an elevated VEGF serum value for the existence of altered P wave dispersion in RA patients was calculated. We estimated the strength of the associations using 95% confidence intervals and a p-value<0.05 was considered statistically significant.
ResultsCohort characteristicsA total of 99 RA patients were included in this study. In addition, 48 control patients matched for age and sex were also included. The mean age in the RA group was 47.1±7.6 years old, and 45.18±13.7 in the control group (p=NS). There were 81 (82%) female patients in the RA group, and 39 (81.3%) female patients in the control group (p=NS). Clinical and demographics characteristics for these two groups are depicted in Table 1. No patient in the control group had ischemic heart disease, two patient had compensated functional class I heart failure, and only one patient had controlled diabetes mellitus type II in the control group. No patient was on amiodarone, or other antiarrhythmic drugs. Only one patient was on beta-blockers as a part of his hypertension treatment. The great majority of the control group were healthy subjects, and only four subjects had compensated cardiovascular risk factors. This latter fact did not alter the statistical significance of the results, since no patient in the control group had altered PWD. Out of the 99 patients with RA, 77% were rheumatoid factor (RF) positive, 41% had osteoarticular erosions, 75% were anti-citrullinated protein antibody (ACPA) positive, 39% had extraarticular manifestation. The mean erythrosedimentation rate-disease activity score28 (ESR-DAS28) was 3.42±1.1, and the mean disease duration was 130.9±102.6 months. The therapeutic management in RA patients at the inclusion in this research is depicted in Table 2.
Clinical and demographic characteristics of RA and Control patients.
| RA patientsn=99 (100%) | Control patientsn=48 (100%) | p value | |
|---|---|---|---|
| Age in years; media±SD | 47.1±7.6 | 45.15±13.7 | 0.2 |
| Female, n (%) | 81 (82%) | 39 (81.3%) | 0.4 |
| Male, n (%) | 12 (12.1%) | 11(22.9%) | 0.2 |
| Weight, kg±SD | 74.1±16.2 | 78.7±14.5 | <0.01 |
| Height, cm±SD | 158.2±16.8 | 161.4±8.2 | 0.28 |
| BMI±SD | 28.6±4.6 | 30.4±5.8 | <0.01 |
| SBP, mmHg±SD | 121.7±15.8 | 121.8±13.8 | 0.20 |
| DBP, mmHg±SD | 75.9±10.1 | 77.1±9.5 | 0.09 |
| Abdominal circumference, cm±SD | 93.4±11.8 | 94.5±16.1 | 0.49 |
| Hip circumference, cm±SD | 104.8±10 | 109.3±14.8 | <0.01 |
| Physical inactivity n (%) | 63 (63.6%) | 20 (41.6%) | <0.01 |
| Arterial hypertension, n (%) | 33 (33.3%) | 1 (2.08%) | <0.001 |
| Diabetes mellitus 2, n (%) | 8 (8.08%) | 1 (2.08%) | 0.14 |
| Heart failure, n (%) | 4 (4.04%) | 2 (4.16%) | 0.28 |
| Stroke, n (%) | 2 (2.02%) | 0 (0.0%) | 0.22 |
| Renal failure, n (%) | 8 (8.08%) | 0 (0.0%) | <0.02 |
SD: standard deviation, BMI: body mass index. SBP: systolic blood pressure. DBP: diastolic blood pressure.
Therapeutic management in RA patients at inclusion in the study.
| Drug treatment | RA group n=99 |
|---|---|
| Hydroxychloroquine n (%) | 28 (28.3%) |
| Glucocorticoids n (%) | 42 (42.4%) |
| Nonsteroidal anti-inflammatory n (%) | 45 (45.5%) |
| Methotrexate n (%) | 80 (80.8%) |
| Leflunomide n (%) | 52 (52.5%) |
| Cyclophosphamide n (%) | 3 (3.03%) |
| Statins n (%) | 8 (8.08%) |
| Angiotensin converting enzyme inhibitors n (%) | 15 (15.2%) |
| Angiotensin II Receptor Blockers n (%) | 12 (12.1%) |
| Calcium Channel Blockers | 2 (2.02%) |
| Betablocking agents | 6 (6.06%) |
| Calcium and Vitamin D | 82 (82.8%) |
| Aspirin | 5 (5.05%) |
The maximal P wave had a mean duration of 100.3±10.2ms in the control group vs. 107.8±16.8ms (p<0.04) in the RA group. The PWD was 25.3±4.9ms in the control group vs. 59.5±14.9ms (p<0.0001) in the RA group. No patient in the control group had altered PWD, defined as ≥38ms, while 94 (95%) patients in the RA group presented it (p<0.0001). Table 3 shows certain clinical characteristics of RA patients grouped according to the presence of normal or altered P wave dispersion.
Clinical characteristics of RA patients grouped according to P wave dispersion.
| Total populationn=99 (100%) | Normal P wave dispersionn=8 (9%) | Altered P wave dispersionn=91 (91%) | p value | |
|---|---|---|---|---|
| Age in years; media±SD | 51±11 | 52±7 | 51±11 | 0.8 |
| Female (%) | 81 (82) | 8 (100) | 79 (86) | 0.7 |
| Male (%) | 18 (18) | 0 | 12 (14) | |
| VEGF levels, pg/ml; media±SD | 51±55 | 20±12 | 56±57 | <0.02 |
| Maximum P wave, ms; media±SD | 107.8±16.8 | 93±12 | 108±16 | 0.07 |
| P wave dispersion, ms; media | 59.5±14.9 (50–70) | 32 (30–35) | 60 (50–70) | <0.001 |
Abbreviations: SD: standard deviation, VEGF: vascular endothial growth factor.
Only one person in the control group had elevated serum value of VEGF vs 24 patients in the RA group [1 (2.4%) vs 24 (25.5%) p<0.0006]. The serum value of VEGF in the control group was 15.2±15.1pg/ml vs 51.1±55.5pg/ml (p<0.001) in RA patients.
The serum value of VEGF in RA patients without altered PWD was 20±12pg/ml vs 56±57pg/ml (p<0.02) in RA patients with altered PWD.
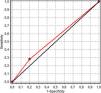
Association of P wave dispersion and increased VEGF serum levelsAn elevated VEGF serum value had a sensitivity of 28% (IC 95% 17–38) (low SnNout), a specificity of 80% (IC 95% 34–100) (high SpPin), a negative predictive value of 6% (IC 95% 0–13), and a positive predictive value of 95% (IC 95% 85–100) in predicting altered PWD in RA patients. Fig. 1 depicts the receiver operating characteristic (ROC) curve of the presence of an elevated VEGF serum value for the existence of altered P wave dispersion in RA patients. The ROC curve was: 54% (IC 95% 33–74).
DiscussionIn the present study, we found significant differences in serum VEGF levels between RA patients who possess altered PWD compared to those RA patients who do not have altered PWD. Moreover, there is a significantly wider P wave duration and dispersion in patients with RA than in control patients. To the best of our knowledge, this is the first study to demonstrate that RA patients who have significantly higher serum levels of VEGF possess an altered PWD. In addition, the presence of higher serum VEGF levels has a high specificity, and a high positive predictive accuracy of the existence of an altered PWD in RA patients.
It was demonstrated that increased P wave duration and dispersion in the 12-leads ECG reflect prolonged atrial conduction time within the right atrium, and between right and left atrium. It also denotes discontinuous, non-homogeneous atrial propagation of sinus impulses in certain patients prone to develop atrial arrhythmias.14–19 Atrial myocardial alterations may occur due to the presence of adverse effects of pro-inflammatory conditions as well.26–29 Diseases and conditions that gradually lead to structural changes in the myocardium and endocardium may cause the appearance of fibrotic changes in the atrial myocardium.26 The anisotropic characteristics caused by a pathological atrial myocardium can play an important role in the creation of conduction delay and reentry circuits in the atrial myocardium.20–22 We have found in the present research a significantly wider P wave duration and dispersion in patients with RA than in control subjects. These findings may indicate the existence of conduction delay in intra-atrial and inter-atrial electromechanical coupling in patients with autoimmune rheumatic disease.
The structural abnormalities in the atrial myocardium and endocardial endothelium are followed by functional changes characterized by synthesis and secretion of different serum biomarkers.28,29 There is an increased expression of adhesion molecules, the release of chemo-attractants, vascular endothelial growth factors and free oxygen radicals.28 VEGF is an important modulator of endothelial function, capillary permeability, and angiogenesis, which is a hallmark of inflammatory activation. VEGF has a mass of 45kDa, and belongs to a family of platelet derived growth factors.30 It was previously demonstrated that the genetic information for VEGF is situated on the short arm of chromosome 6.31,32 Serum VEGF levels are elevated and are directly related with the activity and clinical severity in several different types of autoimmune rheumatic disease.33–36 Therefore, VEGF has a potential pathogenic role with interesting diagnostic value in RA patients. In this respect, Zhan et al.37 had shown significantly increased VEGF levels in their meta-analysis in several types of autoimmune diseases. Additionally, they observed that serum VEGF could distinguish active from inactive SLE, as well as, renal from non-renal SLE. Therefore, they assumed that high serum levels of VEGF reflect pathogenesis and should be considered as a potent hematological biomarker for adequate diagnosis, prognosis, and disease progression in patients with autoimmune diseases.37
Since PWD is considered a noninvasive ECG marker for atrial remodeling and a predictor of atrial arrhythmias development,14–17 and high levels of VEGF are related to atrial myocardium inflammatory and fibrotic changes,26–29 we conceived that they could be related in RA patients. Indeed, we found a 26% incidence of elevated serum levels of VEGF in our 99 patients with RA, and that those RA patients who have significantly higher serum levels of VEGF possess an altered PWD.
Prioreschi et al.38 have shown an increase in physical inactivity in patients with RA compared to healthy controls, although, others have shown no difference.39 We have also found a significantly higher difference of physical inactivity in our RA patients 63 (63.6%) compared to controls 20 (41.6%) p<0.01 in the present study. This sedentary behavior in RA patients may persist due to inadequate control of inflammation, or psychological factors, and lead to serious long-term clinical complications. Sedentary behavior induces a pro-inflammatory and atherogenic state by cellular mechanisms including reduction in lipoprotein lipase activity leading to increased levels of lipids.40 RA patients who are physically inactive experience worse psychological comfort than those who are active.38 In addition, RA patients also exhibit more cardiovascular risk factors, more hospital admissions, lower bone mineral density, and higher bone loss.41–43
It was demonstrated that patients with prolonged atrial and interatrial conduction time manifest augmented P wave duration and dispersion, as well as, other atrial alterations.44–46 Previous studies suggest that interstitial edema in the heart can acutely promote atrial or ventricular arrhythmias by disrupting myocyte intercalated disk rich in cardiac sodium channels (NaV1.5) and slowing cardiac conduction. These disruptions of intercalated disks have been identified in atrial samples from patients with atrial arrhythmias.47–51 A recent well designed animal experimental research52 tested the hypothesis that VEGF-induced vascular leak can acutely increase atrial arrhythmia susceptibility by disrupting intercalated disks and slowing atrial conduction. Mezache et al.52 studied murine hearts with VEGF to acutely predispose otherwise normal hearts to atrial myocardial electrophysiological changes that may develop atrial arrhythmias.52 They observed prolongation of the electrocardiographic P wave, slower atrial conduction, and increased susceptibility to burst pacing induced atrial arrhythmias. They also demonstrated an increased inter-membrane spacing at NaV1.5-rich intercalated disk sites adjacent to gap junctions with transmission electron microscopy.52 These data suggest that VEGF can acutely predispose otherwise normal hearts to atrial arrhythmias by dynamically disrupting NaV1.5-rich intercalated disks and slowing atrial conduction. Therefore, VEGF acutely induces intercalated disks swelling and translocation of sodium channel subunits from these sites, likely generating a substrate for slowed atrial conduction, and atrial arrhythmias in murine hearts closely corresponding to observations in patients with atrial arrhythmias.47,48 Our RA patients with higher serum levels of circulating VEGF had the greater alterations in P wave duration and dispersion. The clinical implication is that RA patients with these two mentioned abnormalities may have developed an atrial substrate that renders them prone to develop atrial arrhythmias. Therefore, these RA patients should have a closer, much longer, and more specific follow-up.
Structural and functional alterations associated with high serum levels of VEGF may lead to tissue damage in chronic inflammatory diseases. Angiogenesis and immune-mediated vascular endothelial cell dysfunction, as well as, persistent inflammation play important pathological roles in patients with autoimmune diseases.26–29 Evidently, VEGF can augment inflammatory processes, resulting in more severe inflammation. Perhaps, although speculative, blocking VEGF production in chronic inflammatory diseases may prevent abnormalities in atrial myocardium diminishing altered P wave dispersion, which may become an important means of ameliorating the severity of some of these RA patients.
LimitationsThe present prospective study has inherent limitations. Although our study population was recruited mainly from a single center, the size of our study population was relatively small. The estimated sample size was 82, and 99 patients with RA were recruited. Since the measurements of the P waves were based on morphological characterization, measurement errors may have occurred to some extent. However, there was an excellent interobserver variability in our research that correlated with a kappa statistic value of 0.90.
Future directionsFurther clinical studies with larger population and longer follow-up are needed to shed light into the mechanism of action, and to find a stronger correlation between VEGF and altered atrial myocardium and PWD, as well as, to resolve inconsistencies and contradictory findings.47,48,53,54 Moreover, the challenge lies in finding new therapeutic strategies that would prevent the onset of cardiovascular diseases by preserving myocardium infrastructure, remodeling of the heart, and endothelial function in order to guide the diagnosis and adequate cardiovascular treatment in RA patients.
ConclusionThere are significantly higher serum levels of circulating VEGF in RA patients who possess altered PWD compared to those RA patients who do not have altered PWD. Moreover, there is a significantly wider P wave duration and dispersion in patients with RA than in control subjects. To the best of our knowledge, this is the first study to demonstrate that RA patients who have significantly higher serum levels of VEGF possess an altered PWD. In addition, the presence of higher serum VEGF has a high specificity, and a high positive predictive accuracy of the existence of an altered PWD in RA patients.
Authors’ contributionEach author has met all four criteria of the International Committee of Medical Journal Editors (ICMJE) for authorship. All authors met the following authorship criteria: substantial contributions to the acquisition, analysis and interpretation of data, contribution to drafting the work and revising it critically, and agreeing to be accountable for all aspects of the work. The final version of this manuscript has been read, analyzed, and approved by all the authors, and they have obtained the required ethical approvals. The authors have given the necessary attention to ensure the integrity of the work and agree to publish this work.
Centurión OA: Conceptualization; Data curation; Formal analysis; Funding acquisition; Roles/Writing-original draft; Writing-review & editing. Abreu P: Conceptualization; Funding acquisition; Formal analysis; Review & editing. Avila-Pedretti G: Investigation; Methodology; Visualization; Review & editing. Cabrera SR: Formal analysis; Funding acquisition; Investigation; Methodology; Review & editing. Martinez de Filártiga MT: Resources; Funding acquisition; Investigation; Methodology; Review & editing, Project administration. Paats A: Investigation; Methodology; Visualization; Review & editing. Torales JM: Investigation; Methodology; Visualization; Validation; Review & editing. Montiel CR: Investigation; Methodology; Visualization; Validation; Review & editing. Chávez CO: Investigation; Methodology; Visualization; Review & editing. García LB: Investigation; Methodology; Visualization; Review & editing. Scavenius KE: Investigation; Methodology; Visualization; Review & editing. Falcón RDP: Investigation; Methodology; Visualization; Review & editing. Candia JC: Investigation; Methodology; Visualization; Review & editing. Meza AJ: Investigation; Methodology; Visualization; Review & editing. Acosta-Colmán I: Conceptualization; Resources; Formal analysis; Project administration; Investigation; Methodology; Visualization; Review & editing.
Ethics approval and consent to participateThe studies were conducted in accordance with the ethical standards of the WMA Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subject and its later amendments, with the approval of the local institutional ethics committee review board. Informed consent was obtained from all the individuals participating in this research. Privacy and anonymity of all patients’ data was granted as there was a code number for every patient file that included all investigations.
FundingThis work was totally supported by a grant (Grant Code; PINV15-699) received from CONACYT (National Commission of Science and Technology) after our research protocol was analyzed by a jury and elected by concourse. This grant was awarded by CONACYT to OAC, PA, and MTM. “The content of this manuscript is the exclusive responsibility of the authors and in no case should it be considered to reflect the opinion of CONACYT.” The funding source had no role, no involvement whatsoever, in the study design; in the collection, analysis and interpretation of data; in the writing of the report; nor in the decision to submit the article for publication.
Conflict of interestNone.
Data availabilityAll datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
We appreciate the technical assistance given by Alberto Marecos, MD, Emilio Lovera, MD, and Oscar A. Lovera, MD, throughout this research. We thank Dr. Angelica Helga Neumann for her constant support in the making of this manuscript, and Miss Felicita Torales for her help with the literature search.