We present the case of a 45-year-old woman, with two-year history of chronic renal insufficiency and proteinuria. A kidney biopsy showed the presence of AA amyloidosis (positive Congo red staining and immunohistochemistry). There was no evidence of amyloid deposits in other organs and there was no underlying disease. AA amyloidosis normally is secondary to chronic inflammatory or infectious diseases. High levels of IL-1, IL-6 and TNF-α play a role in the pathogenesis of amyloidosis and induce the synthesis of serum amyloid A protein (SAA), a precursor of tissue amyloid deposits. We empirically treated the patient with a low dose colchicine. The patient responded well. Colchicine has been used for the treatment of Familiar Mediterranean Fever and related auto-inflammatory diseases. To monitor treatment responses, we measured SAA finding low titers. Soon after treatment onset there were signs of improvement pertaining to proteinuria and stabilization of renal function.
Se presenta el caso de una paciente de 45 años de edad con insuficiencia renal crónica y proteinuria. La biopsia renal demostró una amiloidosis tipo AA con un estudio de extensión negativo para depósitos en otros órganos. No se detectó enfermedad asociada. La amiloidosis tipo AA se asocia habitualmente a una enfermedad crónica inflamatoria o infecciosa. Las interleucinas IL1, IL-6 y TNF son responsables de la síntesis hepática de la proteína sérica amiloide A (SAA), precursor del amiloide que se deposita en los tejidos. Ante la imposibilidad de tratar una amiloidosis sin evidencia de enfermedad subyacente, instauramos empíricamente tratamiento con colchicina con buenos resultados. La colchicina es eficaz para el tratamiento de la fiebre mediterránea familiar y otros procesos inflamatorios. Se midieron niveles de la SAA en sangre que inicialmente fueron muy elevados alcanzándose niveles normales al poco tiempo de tratamiento con mejoría de la proteinuria, manteniéndose estable la función renal.
A 45-year-old woman with stable chronic renal insufficiency and proteinuria was admitted with acute onset of dyspnea and chest pain. Complementary tests demonstrated pulmonary embolism secondary to deep venous thrombosis. Anamnesis revealed chronic malar rash and alopecia. The laboratory test showed a normal blood cells counts, C-reactive protein (PCR) 68mg/dl, erythrocyte sedimentation rate (ESR) 78mm/h, serum creatinine 2.52mg/dl, proteinuria 1.2g/24h. Antinuclear antibodies (ANA) shown positive at low titer (1:80). Anti-dsDNA, anti-mitochondrial, anti-neutrophil cytoplasmic and anticardiolipin antibodies as well as lupic anticoagulant were shown to be negative. C3, C4 and coagulation studies had been shown to be normal. Serum proteins, immunoglobulins and Bence-Jones proteins came back negative.
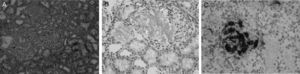
After a dermatological evaluation including cutaneous biopsy the malar rash resulted in rosacea. The renal biopsy revealed AA amyloidosis with Congo red staining and immunohistochemistry positive tests (Fig. 1A–C). A subcutaneous fat tissue and rectal mucosa biopsy showed no signs of amyloid deposition. Bone marrow aspiration, transthoracic echocardiography and full body computer tomography did not identify any signs of amyloid infiltration in other organs. In addition, a genetic study was performed to rule out transthyretin-related hereditary amyloidosis, which came back negative.
(A) Glomerular deposition of amorphous and eosinophilic amyloid. Hematoxylin-eosin stain, original magnification ×20. (B) The glomerular deposits stain positively with Congo red. Typical apple-green birefringence when viewed with polarized light, original magnification ×40. (C) Immunohistochemical stain show amyloid AA deposits within the glomeruli, original magnification ×40.
We diagnosed renal AA amyloidosis without underlying disease and empirically started treatment with colchicine 1mg/day. At the beginning of the treatment, the patient showed SAA levels of 49.1mg/dl. Soon after the initiation, we observed titers lowering down to a normal range. After three months, there was an increase of SAA. So we decided to increase colchicine to 1.5mg/day. Now 12 months later, the SAA levels remain within normal ranges. The proteinuria has improved to levels of 180mg/24h. The patient is asymptomatic and currently has routine checkups.
DiscussionSystemic amyloidosis is a disease resulting in a localized or diffuse deposition of insoluble fibrils in extracellular tissues.1 Immunohistologic study usually reveals AA amyloid (the type of protein associated with chronic inflammation) or AL amyloid (secondary to multiple myeloma).1,6 Both processes produce pro inflammatory cytokines (IL-1, IL6 and TNF-α) which induce hepatic SAA synthesis.3,7 Serum SAA is an acute phase reactant, which after a proteolytic process deposits in various organs in a form of fibrils. Serum SAA titers are associated with the amount of tissue amyloid and the prognosis.2,5
AA amyloidosis occurs associated with chronic arthritis, infections and Familiar Mediterranean Fever.2 In some cases it occurs as an isolated form.2 When amyloidosis is secondary to inflammatory arthritis the treatment consists of using immunosuppressant agents or biological treatments (anti-TNF or IL-1 antagonists).3,7,8 In cases of amyloidosis secondary to Familiar Mediterranean Fever colchicine has been widely used.4
In our patient, there are no signs of underlying disease identifying limited treatment options. Chronic mild proteinuria and renal insufficiency secondary to glomerular sclerosis made complicated the response to treatment, so we decided to measure levels of SAA.5 There are some published cases of renal AA amyloidosis with nephrotic syndrome treated with colchicine. In one of these cases, after using colchicine 1mg per day, the proteinuria completely resolved after four months of treatment and renal function parameters returned to normal as well. A similar case reports rapid improvement of proteinuria down to 50% after 2mg colchicine treatment.9,10
Therefore, we started treatment with colchicine. In subsequent follow-up visits, we observed a dramatic decrease of SAA remaining within normal range during three months of treatment. Later, we observed a new increase of SAA. We decided to increase colchicine to 1.5mg/day reaching normal titers over 12 months.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare no conflict of interest.
FundingThe authors declare no funding from any organization.