To establish the influence of cytokines on anxious-depressive manifestations in fibromyalgia (FM) patients.
Material and methodThe study comprised 56 women (50.5±7.8 years) with FM and 32 healthy female controls (39.65±9.24 years). Psychiatric symptoms were analyzed using the Hamilton Depression Rating Scale (HDRS) and the Hamilton Anxiety Rating Scale (HARS), while pain was assessed by means of a Visual Analog Scale (VAS). Proinflammatory cytokines IL-6, IL-8, IL-10, and TNFα were assayed in serum using the Luminex-xMAP method. The statistical analysis was carried out using the SSPS statistical package.
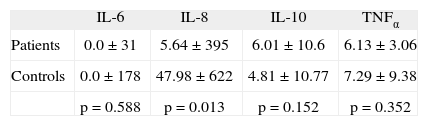
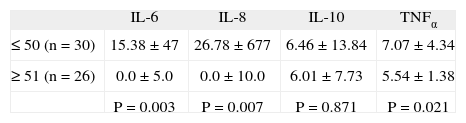
ResultsIL-8 values were significantly lower (p=.013) in patients than in controls. No differences were observed among IL-6, IL-10, and TNFα levels. When comparing cytokine levels with patient age, we observed a significant reduction in IL-6, IL-8, and TNFα in older patients. The values of these cytokines showed no relationship to the anxious-depressive symptoms.
ConclusionsIn our series no differences in serum cytokines were seen between patients with FM and controls, with the exception of a reduction in IL-8 among patients with FM, and which could be attributed to the older age of these patients.
Determinar la influencia de las citocinas en las manifestaciones ansioso-depresivas de pacientes con fibromialgia.
Material y métodoEn el estudio se incluyó a 56 mujeres con fibromialgia (50,5±7,8 años) y 32 mujeres sanas como controles (39,65±9,24 años). Los síntomas psiquiátricos fueron analizados con la Hamilton Depression Rating Scale (HDRS) y la Hamilton Anxiety Rating Scale (HARS), mientras que el dolor fue valorado por medio de una escala visual analógica. Las concentraciones de citocinas proinflamatorias de interleucina (IL)-6, IL-8, IL-10 y factor de necrosis tumoral alfa (TNFα) se midieron en suero mediante Luminex-xMAP. El análisis estadístico se realizó con el programa estadístico SSPS.
ResultadosLas concentraciones de IL-8 fueron estadísticamente más bajas en pacientes que en controles (p=0,013). No se observaron diferencias con respecto a las concentraciones de IL-6, IL-10 y TNFα. Al comparar los valores de citocinas con la edad de los pacientes, se observó una reducción significativa de IL-6, IL-8 y TNFα en los pacientes de mayor edad. No se observó relación entre los valores de estas citocinas y los síntomas ansioso-depresivos.
ConclusionesEn nuestra serie no hay diferencias en las concentraciones séricas de citocinas entre pacientes con fibromialgia y controles, con la excepción de una reducción de IL-8 en los pacientes con fibromialgia que podría ser debida a la mayor edad de los pacientes con respecto a los controles.
Fibromyalgia (FM) is a chronic disease with an uncertain etiopathogenesis. It requires an important amount of attention from the clinical, rheumatologic, psychiatric and general medical perspective, and greatly affects the quality of life of patients due to the intense pain they experience, in addition to the range of somatic and psychiatric symptoms1,2.
Recent findings suggest that cytokines play a key role in FM3. Proinflammatory cytokines IL-2, IL-6, IL-8, IL-10 and TNFα respond to chronic stress by activating the HPA (hypothalamic-pituitary-adrenal) axis and stimulating secretion of corticotrophin-releasing hormone, thus further increasing the secretion of cortisol and causing its concentration in tissues to be higher than in normal situations of stress. These facts could explain the involvement of these cytokines in the development of FM. According to Wallace et al3, the role of these cytokines is as follows: IL-6 is potentially associated with hyperalgesia, depression, stress, fatigue, sympathetic nervous system activation, and promotes substance P secretion; IL-8 is associated with the stimulation of substance P release and mediates sympathetic pain; IL-10 seems to block pain and TNF produces stress, promotes rapid eye movement sleep (REM sleep) and allodynia, regulates substance P expression, induces pain producing excitatory amino acids and stimulates the release of epinephrine and norepinephrine.
Also, a complex interaction of a variety of factors, especially of a genetic, neuroendocrine and psychological nature, among others, has been suggested to play a role in the development of FM4 -psychological factors being particularly significant in a subgroup of patients5,6 who usually show personality traits characterized by the orderliness, control and highly demanding behaviors7, emotional inhibition and alexithymia, rigid defensive mechanisms and anxiety in comparison to healthy controls8. They also have a tendency to action and activity9, and show adjustment difficulties associated to their chronic anxiety and continuous pain.
The main aim of the present study was to measure cytokine levels in the sera of FM patients with anxiousdepressive manifestations and to compare them with those of a control group. A second stage of the study in turn assesses the possible relationship between cytokines and anxious-depressive manifestations.
Material and methodFifty-six women who met the criteria of the American College of Rheumatology for FM10 were studied in the outpatient clinic of a mental health center in Virgen del Rocío University Hospital in Seville (Spain). The patients were referred from the rheumatology service and gave their informed consent to participation in the study. The patients included in our data base FM were 72 (71 women and 1 man), but in this study only 56 consecutive patients fulfill criterion of inclusion evaluated during 2006. All patients suffered from chronic FM with over 10 years of symptoms, and had showed no improvement. They suffered from severe pain, sleep disorders, general physical and psychological alterations and subjective disability symptoms. All patients showed somatic awareness and catastrophic thinking. The mean age of the patients was 50.5 ±7.8 years (range, 30–70 years). The patients showed no medical or psychiatric co-morbidities and were not taking psychoactive substances. During the week preceding the start of the study, they likewise used no steroid derivatives. The psychological evaluation of the patients was conducted using the Hamilton Depression Rating Scale (HDRS)11 and the Hamilton Anxiety Rating Scale (HARS)12. Thirty-two female bone marrow donors were included in the study as healthy controls, with a mean age of 39.65 ± 9.24 years. Any of the controls fulfil the criteria of FM. The age difference with the FM patients had no statistical signification.
IL-6, IL-8, IL-10 and TNF levels in serum samples were measured by means of the Luminex-xMAP method (Luminex Corporation) using the Linco Human Cytokine Panel (Linco Research), following the recommendations of the manufacturer. The samples were obtained in duplicate through vein puncture and stored at −70 °C, between 9.00 and 10.00 am. In order to carry out the statistical analysis, and due to the fact that the detection threshold of the technique is 3.2 pg/ml for all cytokines, all the samples below this value (non-detectable) were assigned a value of 0.
The statistical analysis was performed using the SSPS statistical package. As variances were not homogeneous (Barlett test, p < 0.05), we used the Mann–Whitney/ Wilcoxon test. The results were expressed as median ± interquartile range. Values of p < 0.05 were considered statistically significant.
ResultsAmong the patients with FM (n = 56) and the controls (n = 32), we found no statistically significant differences as regards age. The mean age of the patients was 50.5 ± 7.8 years, versus 39.65 ± 9.24 years among the controls. On analyzing the cytokine levels, a statistically significant reduction in IL-8 values was seen in the patients with FM in comparison to the controls (tabla 1). On comparing interleukin levels with the age of the patients, a significant decrease in IL-6, IL-8 and TNF was noted in older patients (tabla 2).
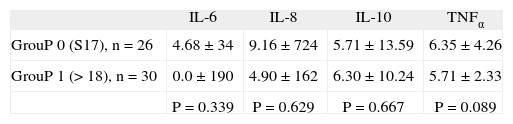
The analysis of cytokines in relation to the HDRS (a scale comprising 17 items where a score ≥ 18 indicates the presence of depression) showed no significant differences (tabla 3). As regards the HARS (a scale comprising 14 items where a score ≥ 11 indicates the presence of anxiety), 53 patients showed anxiety, precluding analysis with cytokines. Fifty of the 53 patients present a score ≥ 25, there for was imposible to make a study of citoquinas levels stratified by anxiety levels.
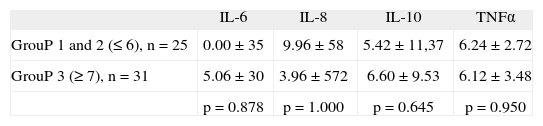
In relation to the Visual Analog Scale for pain, the patients were divided into 3 subgroups, from lower to higher scores (1–4 slight pain, 5–7 moderate pain, > 7 severe pain). No significant differences were observed as regards the cytokine levels among these three subgroups (tabla 4).
DiscussionThe present study reports the influence of cytokines on FM and their association to anxious-depressive manifestations. All the patients studied were women, with a mean age of 50.5 years (range, 25–26). As is well established, FM is more prevalent among women, with a maximum presentation peak around the age of 50.
Measurement of the presence of proinflammatory cytokines in sera showed a statistically significant reduction in IL-8 levels in patients versus the controls. This contrasts with the results reported by other authors, who found elevated levels of this cytokine. Wallace et al3, for instance, reported an increase in IL-8 levels in patients with over two years of symptoms in comparison with patients with fewer than two years of symptoms. However, all our patients had symptoms for more than 10 years. In the other hand, Brazzichi et al13 also find an increase in IL-8 by FM patients, but the target of the study was not analyze the relation between citoquinas and anxiety.
In another study, Uceyler et al14 examined whether there were differences as regards the levels of cytokines in patients with chronic pain. He selected 40 patients with chronic pain (26 with FM) and 40 age- and sex-matched healthy controls. He also selected 15 patients with chronic pain from another center. He observed a reduction in IL-4 and IL-10 in patients with chronic pain. This finding was confirmed in the group of 15 patients with chronic pain. He concluded that chronic pain is related to a lack of activity of cytokine TH2 with analgesic and anti-inflammatory action, which may contribute to the pathogenesis of chronic pain.
Salemi et al15 analyzed the presence of IL-1β, IL-6 and TNF in skin samples from the left deltoid region of 53 patients with FM (30–65 yrs) and 10 age- and sex-matched healthy controls. He found cytokines in the skin of 30% of the patients with FM but in none of the controls, which suggests an inflammatory factor as a cause of pain. This could explain the response to nonsteroidal antiinflammatory drug therapy in a subgroup of patients with FM.
In order to analyze the inflammatory response in patients with FM and investigate the effects of depression on serum cytokines IL-1, IL-2 receptor, IL-6 and IL-8 and the Hamilton Depression Rating Scale (HDRS), Gur et al16 studied 81 patients with FM and 32 healthy controls. IL-1 and IL-6 levels were not significant, but IL-8 and IL-2 receptor levels were significantly higher in patients with FM than in the controls. Moreover, in patients with FM, IL-8 levels were associated to the intensity of pain (R = 0.35; p < 0.01). Thus, IL-8 may play a key role in the onset of pain in patients with FM. However, other studies report normal levels of inflammatory cytokines in patients with chronic fatigue syndrome and FM17. Wallace18, in a review of the literature on FM, suggests that IL-1, IL-6 and IL-8 show altered levels in patients suffering from this disease, and that treatment against these cytokines may be important in its management.
Nevertheless, we should verify whether the significant reduction in IL-8 levels is associated to the duration of the disease and/or to patient age. In this sense, we have observed a statistically significant reduction in IL-6, IL-8 and TNF levels in patients under 50 years of age in comparison to older patients. Also, the controls were 10 years younger than the patients (though the difference was nonsignificant), which could justify the reduction in IL-8 levels among the latter, although, we must considerer the possibility that genetic factors or disease duration, can be responsible of this issue.
ConclusionsSerum proinflammatory cytokine secretion is not altered in patients with FM who show anxious-depressive manifestations and different degrees of pain. However, age could affect the production of these cytokines, particularly that of IL-8, since its response is affected by the older age of the patients (50.5 ± 7.8 years), when compared to healthy controls.