To evaluate the correlation of quantitative anti-dsDNA level with proteinuria levels in patients with lupus nephritis in a tertiary care hospital.
Study designIn this prospective cross-sectional study, 76 patients of newly diagnosed SLE coming to Fatima Memorial Hospital were included in the study period between January 2020 to June 2020. Demographic data such as age, gender, lupus manifestations such as serositis, arthritis, mucocutaneous disease, and neuropsychiatric manifestations were recorded. Quantitative anti-dsDNA was measured by enzyme-linked immunosorbent assay and proteinuria was estimated by 24h urinary protein collection. Data was analyzed by SPSS 23. Association between categorical variables was assessed using chi-square test. For comparison of categorical independent and continuous dependent variable t-test or Mann–Whitney U test was applied.
ResultsThe median age of the cohort was 29 (with inter quartile range – IQR – of 13) years. The female gender comprised of 68 (89.4%) of the cohort population. The median anti-dsDNA level was 54.9 (183.6 IQR) IU, and baseline proteinuria of the cohort was 520mg/dL (1.49 IQR). There was a significant association of anti-dsDNA level with systemic features such as arthritis (p=<0.01), serositis (p=<0.01) and, Raynaud's phenomenon (p=<0.01). NPSLE and mucocutaneous features did not show statistically significant association (p=0.91 and 0.14 respectively). Baseline anti-dsDNA showed a statistically significant correlation with baseline proteinuria levels (p=<0.01).
ConclusionQuantitative anti-dsDNA is directly correlated with nephritis measured as proteinuria, and can be detected even before organ involvement. Hence, it can determine disease course and guide early treatment.
Evaluar la correlación del nivel cuantitativo de anti-dsDNA con los niveles de proteinuria en pacientes con nefritis lúpica en un hospital de tercer nivel.
Diseño del estudioEn este estudio transversal prospectivo se incluyeron 76 pacientes de LES recién diagnosticados que acudieron al Fatima Memorial Hospital en el período de estudio entre enero de 2020 y junio de 2020. Se registraron datos demográficos como edad, sexo, manifestaciones de lupus como serositis, artritis, enfermedad mucocutánea y manifestaciones neuropsiquiátricas. El anti-dsDNA cuantitativo se midió mediante un ensayo inmunoabsorbente ligado a enzimas y la proteinuria se estimó mediante la recogida de proteínas en orina de 24 horas. Los datos fueron analizados por SPSS 23. La asociación entre variables categóricas se evaluó mediante la prueba de χ2. Para la comparación de variable dependiente continua e independiente categórica se aplicó la prueba t o la prueba u de Mann Whitney.
ResultadosLa mediana de edad de la cohorte fue de 29 años (con rango intercuartil – IQR – de 13). El género femenino comprendía 68 (89,4%) de la población de la cohorte. El nivel medio de anti-dsDNA fue 54,9 (183,6 IQR) UI, y la proteinuria basal de la cohorte fue de 520mg/dL (1,49 IQR). Hubo una asociación significativa del nivel de anti-dsDNA con características sistémicas como artritis (p=<0,01), serositis (p=<0,01) y fenómeno de Raynaud (p=<0,01). El NPSLE y características mucocutáneas no mostraron asociación estadísticamente significativa (p=0,91 y 0,14, respectivamente). El anti-dsDNA basal mostró una correlación estadísticamente significativa con los niveles basales de proteinuria (p=<0,01).
ConclusiónEl anti-dsDNA cuantitativo se correlaciona directamente con la nefritis medida como proteinuria y puede detectarse incluso antes de la afectación de órganos; por lo tanto, puede determinar el curso de la enfermedad y orientar el tratamiento temprano.
Systemic lupus erythematosus (SLE) is a multi-systemic autoimmune disorder that can cause wide variety of clinical as well as laboratory manifestations.1 Clinical presentation of this prototype autoimmune connective tissue disease range from mild mucocutaneous disease to multi system involvement comprising of nephritis, neuropsychiatric (NPSLE), and haematological involvement.2 SLE is diagnosed on clinical basis in settings of positive autoimmune antibodies, however, classification criteria that have evolved over decades are commonly used for diagnosis as well.3 American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) proposed in 2019 ACR/EULAR classification criteria for SLE that requires a constellation of clinical plus serological evidence of lupus for classification and diagnosis.4
Lupus nephritis occurring in at-least half or more of lupus population is the most important systemic involvement and the play a main role in treatment plan.5 Nephritis in the form of proteinuria can lead to rapidly progressive glomerulonephritis with resulting end stage kidney disease in months if untreated.6 Whatever the manifestation is, proteinuria associated with SLE has a poor prognosis.7
Another hallmark of SLE is number of auto antibodies against different intracellular components such as nucleic acid named ANA, and DNA named anti-dsDNA etc.8 Accumulating evidence suggests that in addition to forming immune complexes and triggering complement activation, anti-dsDNA antibodies contribute to the pathogenesis of lupus nephritis through binding, either directly or indirectly, to cross-reactive antigens or chromatin materials.9 Anti-dsDNA antibodies are lupus specific antibodies that not only can precede the clinical onset of disease by at least 2 years, but act also as a marker of disease activity and a herald of lupus flare.10 Anti-dsDNA levels rise approximately10 years before the onset of organ involvement especially renal disease.11
The relationship of urinary protein with quantitative levels of anti-dsDNA is not studied widely in our population. We aim to seek out correlation of the two variables at the baseline of the disease, along with correlation with other clinical features of lupus.
Patients and methodsThis study was descriptive cross-sectional study over the period of 6 months conducted in Department of Rheumatology at Fatima Memorial Hospital, Lahore. A total of 76 already diagnosed patients of SLE according to ACR/EULAR 2019 were enrolled through universal sampling technique. Informed consent was taken from all patients at time of enrolment. Patients were reviewed for demographic features such as age, and gender; clinical features of lupus such as skin rash, oral ulcers, serositis, arthritis, neuropsychiatric SLE(NPSLE), Raynaud's phenomenon; and laboratory parameters comprising of complete blood count (CBC), proteinuria estimation by either 24h urinary proteins collection or spot urine sample used for protein to creatinine ratio, ANA by indirect immunofluorescent assay (IFA), quantitative anti-dsDNA measured by Crithidia Luciliae immunofluorescent assay. The levels of anti-dsDNA levels below 30IU/ml were considered as normal, between 31 and 75IU/ml were considered indeterminate, and values above 75IU/ml were considered as positive – as per reference ranges of hospital laboratory. Proteinuria was defined as having 24-h urinary protein of at-least 500mg/day, or spot urinary protein to creatinine ration of more than 0.5. Patients with age less than 17 years, pregnant females, patients with known diabetes and/or hypertension with nephropathy and patients with diagnosis of mixed connective tissue disease, overlap syndrome and anti-phospholipid antibody syndrome (APLAS) were excluded from the study. The study was approved by Institutional Review Board (IRB) for any ethical issue at Fatima Memorial Hospital.
Data was entered and analyzed in SPSS version 23. Categorical variables were presented in form of frequency and percentages. Metric variables were presented in form of mean plus minus standard deviation or median into IQR depending upon the distribution of the data. Association between categorical variables were assessed using chi-square test. For comparison of categorical independent and continuous dependent variable t test or Mann–Whitney U test was applied.
ResultsMedian age of study population was 29 (13) years, with males being of younger age than females (21.50 (8 IQR) vs 30.0 (13 IQR) years) respectively. Female gender comprised of 68 (84.9%) of the total population. Mean duration of disease was 56.68±31.48 months (median 48 months; minimum 12 and maximum 144 months). Oral ulcers were present in 43 (53.1%) patients. malar and discoid rash was found to be present in 37 (45.7%) and 11 (13.6%) patients respectively, while vasculitic rash was found to be in 9 (11.1%) patients. Raynaud phenomenon was present in 29 (35.8%) patients, arthritis and serositis was present in 41 (50.6%) and 8 (9.9%) patients respectively. NPSLE was found to be present in 7 (8.6%) patients. ANA was positive in 55 (67.9%) patients at a titre of 160. Median anti-dsDNA level was 54.9 (183.6) IU, baseline proteinuria was 520mg/dL (1.49 IQR). There was a significant association of anti-dsDNA level with systemic features such as arthritis (p=<0.01), serositis (p=<0.01), and Raynaud's phenomenon (p=<0.01). Neuropsychiatric manifestations and mucocutaneous features like oral ulcers, malar rash and discoid rash did not show statistically significant association (p=0.91, 0.14, 0.19 and 0.36 respectively). It shows that CNS manifestations/NPSLE does not have any association with level of anti-dsDNA (Tables 1 and 2).
Baseline characteristics of cohort.
| Characteristic | Descriptive |
|---|---|
| Age* | 29 (13) |
| Male | 21.50 (8) |
| Female | 30.0 (13) |
| Gender** | |
| Male | 8 (9.9%) |
| Female | 68 (84.9%) |
| Duration of disease (months) | 48.0 (36) |
| Systemic manifestations | |
| Oral ulcers | 43 (53.1%) |
| Malar rash | 37 (45.7%) |
| Discoid rash | 11 (13.6%) |
| Vasculitic rash | 9 (11.1%) |
| Raynaud's phenomenon | 29 (35.8%) |
| Arthritis | 41 (50.6%) |
| Serositis | 8 (9.9%) |
| NPSLE | 7 (8.6%) |
NPSLE: neuropsychiatric SLE.
Correlation of level of anti-dsDNA with systemic manifestations of lupus.
| Clinical manifestation | Median value of anti-dsDNA (IU) | p value | |
|---|---|---|---|
| Present | Absent | ||
| Arthritis | 149 (182) | 19 (62) | <0.01 |
| Serositis | 267 (1918) | 41 (150) | <0.01 |
| NPSLE | 19 (186) | 54 (182) | 0.918 |
| Oral ulcers | 60 (183) | 41 (133) | 0.14 |
| Malar rash | 94 (218) | 41 (183) | 0.19 |
| Discoid rash | 14 (139) | 58 (184) | 0.36 |
| Raynaud's phenomenon | 161 (199) | 28 (119) | <0.01 |
()=IQR for median values.
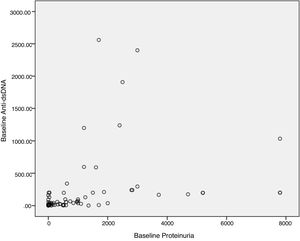
Baseline anti-dsDNA showed statistically significant correlation with baseline proteinuria levels when assessed both as a continuous variable (p=<0.01, Fig. 1), and categorical variable (p=<0.01). Table 3 elaborates the association of anti-dsDNA with proteinuria with tabulated as a categorical variable as following: proteinuria less than 500mg/dL was categorized as Trace, 1+ proteinuria equals from 500mg/dL to 1000mg/dL, 2+ proteinuria equal more than 1000mg/dL to <2000mg/dL, and 3+ equal to >2000mg/dL.
Correlation of anti-dsDNA levels with baseline proteinuria.
| Anti-dsDNA level | Proteinuria level | p value | |||
|---|---|---|---|---|---|
| Trace | 1+ | 2+ | 3+ | ||
| <75 | 17 (42.5) | 13 (32.5) | 6 (15) | 4 (10) | <0.01 |
| 75–200 | 5 (27.8) | – | 7 (38.9) | 6 (33.4) | |
| >200 | 3 (16.7) | – | 1 (5.6) | 14 (.77.9) | |
| Total | 25 (32.9) | 13 (17.1) | 14 (18.4) | 24 (31.6) | |
[n (%)], baseline proteinuria is categorized as follows: Trace: less than 500mg/dL; 1+: <1000mg/dL; 2+: >1000 to <2000mg/dL; 3+: >2000mg/dL.
Anti-dsDNA is not only a specific marker of lupus disease activity but also a predictor of many systemic features of lupus. Our study showed a significant association of quantitative anti-dsDNA with baseline proteinuria, which in itself is a feature of severity of nephritis. In addition, anti-dsDNA also showed strong association with serositis, arthritis, and Raynaud's phenomenon.
Certain important clinical aspects of this study are worth discussing. The study results are concordant with many international studies across the globe. Duarte-García et al. who analyzed John Hopkin Lupus cohort, had found a significant association of anti-dsDNA titres and low complements with proteinuria.12 Both these entities are markers of lupus activity and is translated as proteinuria clinically. Engli et al. had also concluded similar association of severity of proteinuria both as spot protein to creatinine ratio (PC ratio) and on dipstick method with anti-dsDNA levels.13 They also proposed a unique association of severity of proteinuria with site of immune complex deposition in a glomerulus, which gave an insight on pathophysiology of proteinuria itself. Fabrizio et al. showed significant association of anti-dsDNA with both nephritis and serositis, similar to our cohort.14 Gheita et al. concluded significant association of anti-dsDNA with systemic features such as arthritis, and with over all disease activity and damage.15 Their study, however, did not show any significance with nephritis or proteinuria. Yang et al. did not show any significant association of anti-dsDNA with proteinuria or over all disease activity of Lupus per se, but a combination of anti-C1q and anti-dsDNA showed significance.16 Although there is a discordance of our results from the last two mentioned studies, but both the studies showed significant association with lupus disease activity or at least some part of it, which is a concordant finding.
Saigal et al. also showed significant association of anti-dsDNA with not only proteinuria but with over all lupus disease activity as SLEDAI, including arthritis, and serositis, concordant to our finding.17
Roshila et al. found out significant association of anti-dsDNA with SLEDAI in a paediatric lupus population.18 The association was not significant with clinical features but only lab parameters, further narrowing the association to include proteinuria as a significant association.
No dedicated study on association of anti-dsDNA with proteinuria was found locally, however, certain studies do show similar associations of anti-dsDNA with increased lupus activity. Most of the local studies reported the clinical manifestations and laboratory parameters of lupus.19–23 Although no direct association was evaluated in these studies, the nearly equal prevalence of nephritis and anti-dsDNA can point to possible association.24
Certain characteristics of our study increase its strengths. Firstly, it is the first local and study which directly correlates these two important laboratory parameters. It was a prospective study ad evaluation not only included laboratory parameters but also covered certain important clinical parameters including uncommon features such as vasculitic rash and Raynaud's phenomenon.
There are however certain limitations of our study. We did not evaluate these laboratory parameters with lupus activity at baseline. We did not include other features of nephritis such as hematuria, pyuria and cellular casts which are worth mentioning features of nephritis, and lastly, we did not evaluate the effect on outcome of these two laboratory measures, which will add useful information in clinical management. All patients were not undergone renal biopsy, so their results are not mentioned. We propose, larger prospective cohort study focusing on extended clinical manifestation, lupus activity and effect on outcomes for further studies.
SLEDAI (SLE disease activity index) includes scores of all the above mentioned parameters including proteinuria and anti-dsDNA, both of which contribute to disease severity index, hence SLEDAI is not separately mentioned here. Serum complements not mentioned in this study, although these values are low when disease is active.
To conclude, anti-dsDNA is strongly associated with proteinuria and lupus nephritis, arthritis, and serositis, and is a sensitive marker of lupus activity, that helps in predicting the outcomes of lupus in form of organ involvement.
Authors’ contributionsSadia Asif: collaborated on study design and participated in manuscript writing.
Asadullah Khan: participated in collection. Had full access to the entire data in the study and takes full responsibility for the integrity of the data.
Sarmad Zahoor: initiated the idea and participated in manuscript writing.
Naveed Lashari: participated in collection. Had full access to the entire data in the study and takes full responsibility for the integrity of the data.
Muhammad Haroon: Supervised whole study. Reviewed the article, helped in data collection, follow up of the patient and reviewed the article as well.
Afshan Khanum: Helped in data collection, follow up of the patient and reviewed the article as well.
Ethical approvalThe study protocols and informed consent documents were approved by the Institutional Bioethics Review Committee (IBRC).
FundingThere is no role of any funding agency in this study.
Conflict of interestNone declared.
Special thanks to Miss Afshan Khanum and Mr. Ahsan for their cordial help in different aspects of the research.