To develop a multivariable clinical prediction model for the requirement of aggressive immunosuppression with cytostatics, based on simple clinical record data and lab tests. The model is defined in accordance with the result of the kidney biopsies.
MethodsRetrospective study conducted with data from patients 16 years and older, with SLE and nephritis with less than 6 months of evolution. An initial bivariate analysis was conducted to select the variables to be included in a multiple logistic regression model. Goodness of fit was evaluated using a Hosmer–Lemeshow test (H–L) and the discrimination capacity of the model by means of the area under the ROC (AUC) curve.
ResultsData from 242 patients was gathered; of these, 18.2% (n=44) did not need an addition of cytostatics according to the findings of their kidney biopsies. The variables included in the final model were 24-h proteinuria, diastolic blood pressure, creatinine, C3 complement and the interaction of hematuria with leukocyturia in urinary sediment. The model showed excellent discrimination (AUC=0.929; 95% CI=0.894–0.963) and adequate calibration (H–L, P=.959).
ConclusionIn recent-onset LN patients, the decision to use or not to use intensive immunosuppressive therapy could be performed based on our prediction model as an alternative to kidney biopsies.
Desarrollar un modelo multivariado de predicción clínica basado en datos sencillos de la historia clínica y de las pruebas de laboratorio de la necesidad de inmunosupresión intensiva con citostáticos, definida de acuerdo con el resultado de la biopsia renal, en pacientes con LES y nefritis de reciente inicio.
MetodologíaSe realizó un estudio retrospectivo en 2 hospitales de tercer nivel en el que se recolectó información de pacientes mayores de 16 años con LES y nefritis de menos de 6 meses de evolución. Se realizó un análisis bivariado inicial para seleccionar las variables a incluir en un modelo de regresión logística múltiple. Se evaluó la bondad de ajuste por medio del estadístico de Hosmer–Lemeshow (H-L) y la capacidad de discriminación del modelo mediante área bajo la curva ROC (AUC).
ResultadosSe recolectó información de 242 pacientes, de los cuales el 18,2% (n=44) no necesitaba tratamiento intensivo con citostáticos de acuerdo con los hallazgos de la biopsia renal. Las variables incluidas en el modelo final fueron proteinuria en 24h, presión arterial diástolica, creatinina, complemento C3 y la combinación de hematuria con leucocituria presentes en el análisis del sedimento urinario. El modelo mostró una excelente capacidad de discriminación (AUC=0,929; IC del 95%=0,894–0,963) y adecuada calibración (H-L=0,959).
ConclusiónEn pacientes con NL de reciente inicio, la decisión de usar o no terapia inmunosupresora intensiva podría ser realizada sobre la base de nuestro modelo de predicción como una alternativa a la biopsia renal.
One of the most frequent severe complications of systemic lupus erythematosus (SLE) is lupus nephritis (LN). One third of all adults with SLE present LN when diagnosed with their disease and up to two-thirds of them may present this complication during the disease. LN has various forms of histological and clinical presentations with some degree of correlation among them. The dominant expression is proteinuria, many times within a nephrotic range. This may be associated with hypertension, varying levels of kidney function impairment and urinary sediment alterations, especially hematuria and the presence of cellular casts.1
Currently, it is an accepted practice to perform a kidney biopsy as a standard reference to confirm the type of nephritis, and specifically to obtain information related to treatment and prognosis of the disease.2,3 In accordance with International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 Classification, LN may be classified into six classes: minimal mesangial (I), proliferative mesangial (II), focal proliferative (III), diffuse proliferative (IV), membranous (V), an advanced sclerosing (VI).4
A class I or II LN patient's treatment is usually with medium doses of steroids and antiproteinuric measures (i.e. ACE), while those with proliferative forms (III or IV), pure membranous (V), or combinations (III+V, or IV+V) will require additional immunosuppressant therapy aiming to achieve the remission of an active inflammatory process and to reduce the probability of relapses and long term kidney failure.2,3 The treatment of patients with class VI nephritis does not involve cytostatics, but this histological class is rarely found in patients who have been recently diagnosed with kidney disease.
Kidney biopsy is an invasive procedure which increases costs and it is not free from the possibility of potentially serious adverse events.5–7 Some groups propose a more conservative use of biopsies, treating LN patients based on clinical presentation patterns and reserving kidney biopsies for those cases when it is not possible to identify a clear clinical presentation, when a patient does not improve or when there is a suspicion that the cause is other than LN.8,9 Even though many professionals strongly base their practice on biopsies to predict long term outcome, others consider that histological findings actually offer little or no additional information to the one that can be obtained with clinical and laboratory variables.10–12
Though some works have shown certain types of correspondence between clinical and laboratory LN manifestations with histological kidney alterations, these findings have not been constant and they are usually based on univariate analyses.13–17 Our objective was to develop a multivariate prediction model for probability of needing intensive immunosuppression according to the result of kidney biopsies, based on simple data obtained from clinical records and lab tests on SLE patients with recent-onset LN.18,19
Patients and MethodsDesign of the StudyRetrospective cohort study to develop a diagnostic predictive model with patients evaluated between 2004 and 2014.
ParticipantsClinical records of all patients diagnosed with SLE of any age and any gender who were treated from January 2004 to October 2014 in two third level hospitals from Medellin Colombia were revised. To be eligible for inclusion, patients were required to have had an available result of a kidney biopsy performed within the first 6 months since onset of symptoms or laboratory abnormalities attributable to nephritis. The 1982 American College of Rheumatology classification criteria, which were updated in 1997, were used to diagnose SLE.20,21 All the data were collected in standard case report forms from clinical records by the investigators, independently of any result on kidney biopsy report.
OutcomeThe outcome variable was the indication to use intensive immunosuppressive treatment with cytostatics (either cyclophosphamide or mycophenolate mofetil) depending on the kidney biopsy report (Class III, IV, V, III+V, or IV+V LN, versus Class I, II or VI LN). The median time between the first tests and the performing of a biopsy was 1 week, and the outcome was independent from the predictor variables considered. Performing a biopsy was not conditioned on the results of the candidate predictor variables. In every SLE patient with possible nephritis, a kidney biopsy was performed in accordance with guidelines and common practices unless there was a clinical contraindication. In both hospitals a cutoff of 500mg of proteinuria is used to order a kidney biopsy.
PredictorsWhen kidney biopsy was indicated during outpatient or inpatient care, the initial values from medical records for the following candidate predictor variables were obtained: age in years, gender, black race or another, systolic and diastolic blood pressure (mm Hg), creatinine (mg/dl), blood urea nitrogen (BUN, mg/dl), potassium (mEq/l), hematuria, leukocyturia, red blood cell (RBC) casts and white blood cell (WBC) casts (maximum number of erythrocytes, leukocytes, RBC casts or WBC casts per high power field, respectively), proteinuria (mg in 24h), C3 complement (mg/dl), C4 complement (mg/dl), anti-DNA antibodies (positive ≥1/80), hemoglobin (g/dl), platelets (μl−1), serum albumin (g/dl) and the disease activity measured by SLEDAI score (0–105). These variables were selected based on an extensive literature review1,13–17,22–27 and our own clinical experience. A variable called “modified SLEDAI” was created excluding the kidney-related parameters (proteinuria, pyuria, hematuria and cellular casts) platelets, anti-DNA and complement to avoid the collinearity with these same variables measured individually. The first values recorded in medical records were consistently obtained. Almost all of these variables corresponded to laboratory results; therefore, their results and definitions were independent among them.
Sample SizeWe expected, in accordance with the literature review, that 15%–20% of the patients had a form of LN which would not need an addition of intensive therapy with cytostatics. Since the most relevant clinical outcome is needing to use cytostatics, sample size calculation was focused on that outcome. According to the rule of at least 10 outcomes for each independent variable, a sample size of approximately 300 patients was calculated, considering that the outcome would be presented for about 240 patients and this allowed us to consider all of those predictors previously explained.28,29
Missing DataMissing values were not imputed. The variables with more than 10% missing data were not considered for the model. All analyses were based on the cases with complete data. It was assumed that the missing data of predictor variables occurred completely at random.
Statistical Analysis MethodsQuantitative variables were described as means or medians with their respective measure of dispersion depending on data distribution; and qualitative variables as absolute number and percentages. The relation between independent variables and the outcome was explored using Student's t test and Mann–Whitney U test depending on the distribution of the quantitative variables, and for categorical variables Chi square or Fisher's exact test were used according to the expected values in the cells.
We conducted univariate logistic regression with each one of the independent variables previously defined. Monotony of quantitative variables was graphically evaluated using a non-parametric locally weighted regression model (LOWESS),30 and accordingly, its inclusion as continuous or transformed dummy variables was defined.
For each univariate logistic regression analysis the Odds Ratio (OR), the Wald test, the R2 and the corresponding ROC curve were analyzed. Thus, the discrimination and calibration ability of each one of the candidate predictor variables was explored and for the final model those with a P value<.25 on the Wald test and/or an AUC>0.75 were considered.
Two interaction terms were built based on clinical considerations: between proteinuria and creatinine and also between hematuria and leukocyturia. Then, we compared the log likelihood of each complete model with the interaction term being studied versus the respective nested model which presumes the absence of interaction, using the likelihood ratio test with a P value<.10 considered as statistically significant.31
Afterwards, all the candidate variables proposed plus the significant interaction terms were included in a multivariate logistic regression model, and different alternative models with fewer number of variables were explored seeking the one that would offer the best performance with the most reasonable number of predictors. For this selection, manual comparisons of the discrimination and calibration properties of each model were made using ROC curves and Hosmer–Lemeshow goodness-of-fit tests, respectively. We were always guided by clinical criteria and the principle of parsimony to select the final model. In addition, the presence of collinearity was discarded using the correlation matrix of the independent variables expecting values lower than 0.532 and using the variance inflation factor method (VIF) expecting values lower than 10.33 We decided in each case how much the coefficients and the prediction ability were affected by the variables that showed high collinearity. Internal validity was assessed with a bootstrapping procedure in 200 samples drawn without replacement from the original population. After obtaining the model with the best performance and its respective predictor variables, a spreadsheet was designed to obtain the probability of the outcome in accordance with the individual values of the variables included in the logistic regression equation.
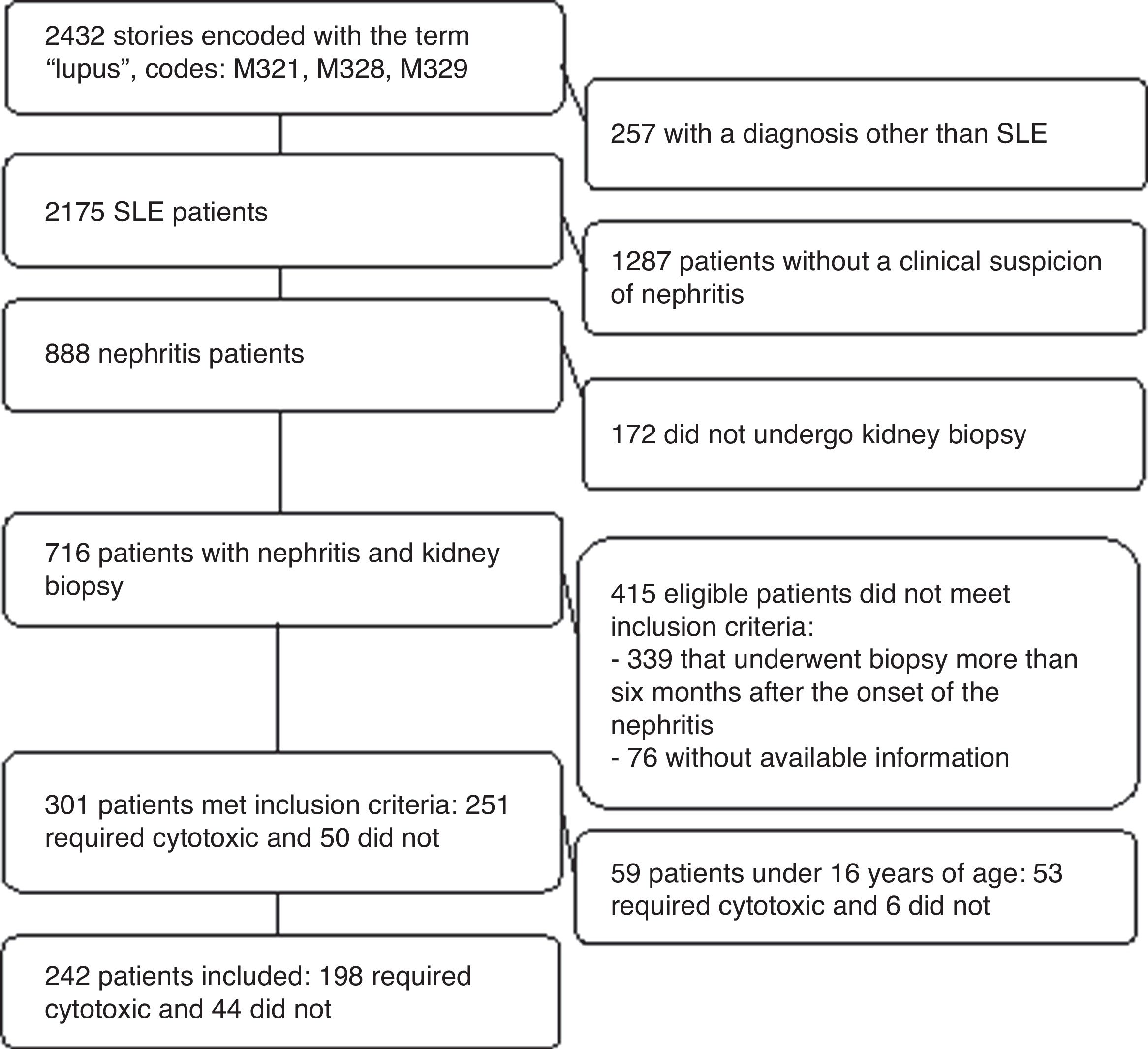
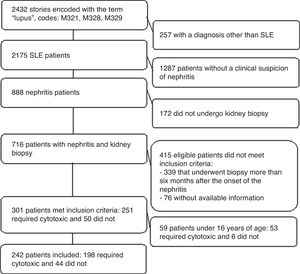
ResultsParticipantsA total of 301 clinical records were studied and 242 patients older than 16 years were included. Those younger than 16 years (n=59 children) were excluded because of considerations regarding their representativeness, the predictive capacity of the model and the external validity of the results. Furthermore, just 6 of these minors did not need immunosuppression (Fig. 1).
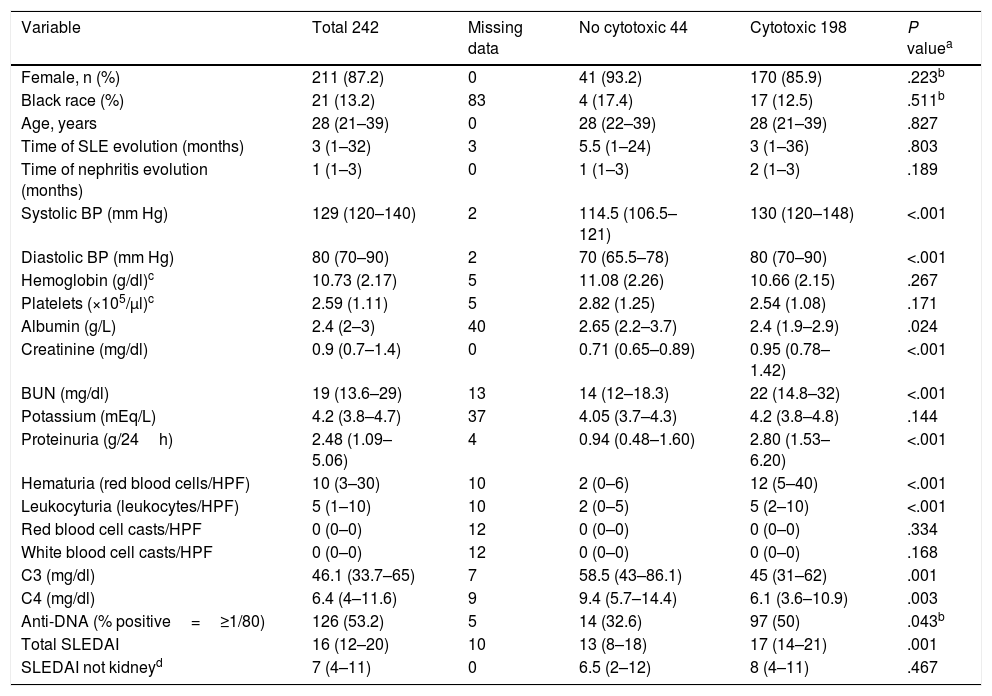
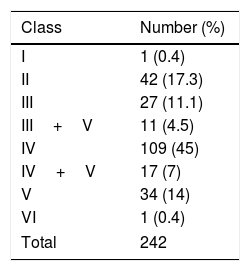
Table 1 shows the clinical and demographic characteristics of patients included depending on the needing for intensive immunosuppression. The median time of SLE evolution was 3 (IQR=1–32) months, the median time of evolution of symptoms attributable to nephritis was 1 (1–3) month and the median time elapsed between nephritis and the performance of the biopsy was 1 (1–4) week. The median SLEDAI score was 16 (12–20) and there were missing data on race (n=83), albumin (n=40) and potassium (n=37). There were 198 patients with proliferative forms of nephritis that required the addition of cytostatics. The different classes of nephritis found are shown in Table 2.
Characteristics of the Patients Included in Accordance With the Needing for Aggressive Immunosuppression.
| Variable | Total 242 | Missing data | No cytotoxic 44 | Cytotoxic 198 | P valuea |
|---|---|---|---|---|---|
| Female, n (%) | 211 (87.2) | 0 | 41 (93.2) | 170 (85.9) | .223b |
| Black race (%) | 21 (13.2) | 83 | 4 (17.4) | 17 (12.5) | .511b |
| Age, years | 28 (21–39) | 0 | 28 (22–39) | 28 (21–39) | .827 |
| Time of SLE evolution (months) | 3 (1–32) | 3 | 5.5 (1–24) | 3 (1–36) | .803 |
| Time of nephritis evolution (months) | 1 (1–3) | 0 | 1 (1–3) | 2 (1–3) | .189 |
| Systolic BP (mm Hg) | 129 (120–140) | 2 | 114.5 (106.5–121) | 130 (120–148) | <.001 |
| Diastolic BP (mm Hg) | 80 (70–90) | 2 | 70 (65.5–78) | 80 (70–90) | <.001 |
| Hemoglobin (g/dl)c | 10.73 (2.17) | 5 | 11.08 (2.26) | 10.66 (2.15) | .267 |
| Platelets (×105/μl)c | 2.59 (1.11) | 5 | 2.82 (1.25) | 2.54 (1.08) | .171 |
| Albumin (g/L) | 2.4 (2–3) | 40 | 2.65 (2.2–3.7) | 2.4 (1.9–2.9) | .024 |
| Creatinine (mg/dl) | 0.9 (0.7–1.4) | 0 | 0.71 (0.65–0.89) | 0.95 (0.78–1.42) | <.001 |
| BUN (mg/dl) | 19 (13.6–29) | 13 | 14 (12–18.3) | 22 (14.8–32) | <.001 |
| Potassium (mEq/L) | 4.2 (3.8–4.7) | 37 | 4.05 (3.7–4.3) | 4.2 (3.8–4.8) | .144 |
| Proteinuria (g/24h) | 2.48 (1.09–5.06) | 4 | 0.94 (0.48–1.60) | 2.80 (1.53–6.20) | <.001 |
| Hematuria (red blood cells/HPF) | 10 (3–30) | 10 | 2 (0–6) | 12 (5–40) | <.001 |
| Leukocyturia (leukocytes/HPF) | 5 (1–10) | 10 | 2 (0–5) | 5 (2–10) | <.001 |
| Red blood cell casts/HPF | 0 (0–0) | 12 | 0 (0–0) | 0 (0–0) | .334 |
| White blood cell casts/HPF | 0 (0–0) | 12 | 0 (0–0) | 0 (0–0) | .168 |
| C3 (mg/dl) | 46.1 (33.7–65) | 7 | 58.5 (43–86.1) | 45 (31–62) | .001 |
| C4 (mg/dl) | 6.4 (4–11.6) | 9 | 9.4 (5.7–14.4) | 6.1 (3.6–10.9) | .003 |
| Anti-DNA (% positive=≥1/80) | 126 (53.2) | 5 | 14 (32.6) | 97 (50) | .043b |
| Total SLEDAI | 16 (12–20) | 10 | 13 (8–18) | 17 (14–21) | .001 |
| SLEDAI not kidneyd | 7 (4–11) | 0 | 6.5 (2–12) | 8 (4–11) | .467 |
Continuous data presented as median (Interquartile range) unless otherwise explained.
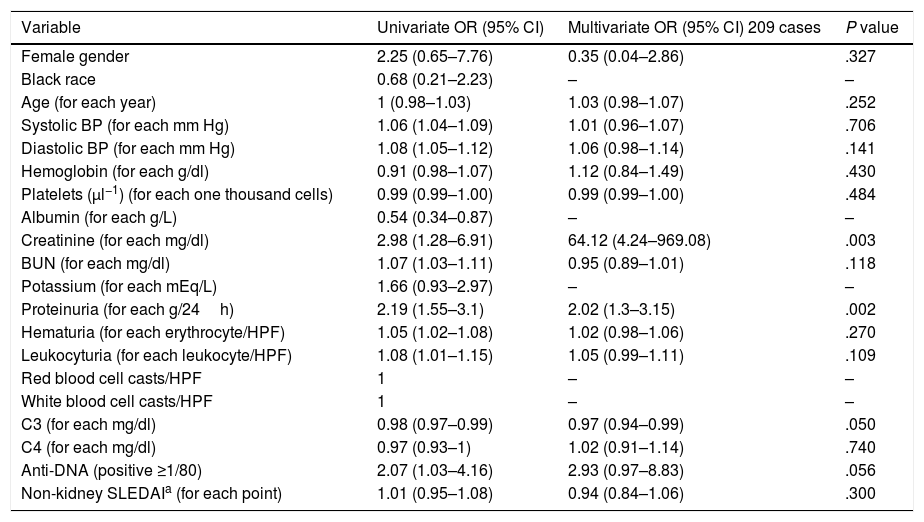
Table 3 shows the univariate and multivariate logistic regression between each candidate predictor and the outcome variable. For an initial analysis, race, albumin and potassium were excluded because of the high proportion of missing data. The RBC and WBC cellular casts variables had medians of 0 (0–0) in both groups; therefore, they were also omitted on the multivariate logistic regression. Possible collinearity between BUN and creatinine (r=0.651, VIF=1.91), between systolic BP and diastolic BP (r=0.788, VIF=2.92) and between complement C3 and C4 (r=0.713, VIF=2.46) was detected. Therefore, BUN, systolic BP and C4 were excluded and kept the other because they presented a higher univariate OR. Then, for the final model, age, diastolic BP, creatinine, proteinuria, complement C3, hematuria, leukocyturia and anti-DNA were considered; with complete data for 223 cases. The variables that showed coefficients with statistical significance were diastolic BP, creatinine, proteinuria and C3. The model with these four variables had an AUC=0.909 (95% CI=0.869–0.949) and a P value on Hosmer–Lemeshow test of .999.
Univariate and Multivariate Logistic Regression Model for Candidate Predictors.
| Variable | Univariate OR (95% CI) | Multivariate OR (95% CI) 209 cases | P value |
|---|---|---|---|
| Female gender | 2.25 (0.65–7.76) | 0.35 (0.04–2.86) | .327 |
| Black race | 0.68 (0.21–2.23) | – | – |
| Age (for each year) | 1 (0.98–1.03) | 1.03 (0.98–1.07) | .252 |
| Systolic BP (for each mm Hg) | 1.06 (1.04–1.09) | 1.01 (0.96–1.07) | .706 |
| Diastolic BP (for each mm Hg) | 1.08 (1.05–1.12) | 1.06 (0.98–1.14) | .141 |
| Hemoglobin (for each g/dl) | 0.91 (0.98–1.07) | 1.12 (0.84–1.49) | .430 |
| Platelets (μl−1) (for each one thousand cells) | 0.99 (0.99–1.00) | 0.99 (0.99–1.00) | .484 |
| Albumin (for each g/L) | 0.54 (0.34–0.87) | – | – |
| Creatinine (for each mg/dl) | 2.98 (1.28–6.91) | 64.12 (4.24–969.08) | .003 |
| BUN (for each mg/dl) | 1.07 (1.03–1.11) | 0.95 (0.89–1.01) | .118 |
| Potassium (for each mEq/L) | 1.66 (0.93–2.97) | – | – |
| Proteinuria (for each g/24h) | 2.19 (1.55–3.1) | 2.02 (1.3–3.15) | .002 |
| Hematuria (for each erythrocyte/HPF) | 1.05 (1.02–1.08) | 1.02 (0.98–1.06) | .270 |
| Leukocyturia (for each leukocyte/HPF) | 1.08 (1.01–1.15) | 1.05 (0.99–1.11) | .109 |
| Red blood cell casts/HPF | 1 | – | – |
| White blood cell casts/HPF | 1 | – | – |
| C3 (for each mg/dl) | 0.98 (0.97–0.99) | 0.97 (0.94–0.99) | .050 |
| C4 (for each mg/dl) | 0.97 (0.93–1) | 1.02 (0.91–1.14) | .740 |
| Anti-DNA (positive ≥1/80) | 2.07 (1.03–4.16) | 2.93 (0.97–8.83) | .056 |
| Non-kidney SLEDAIa (for each point) | 1.01 (0.95–1.08) | 0.94 (0.84–1.06) | .300 |
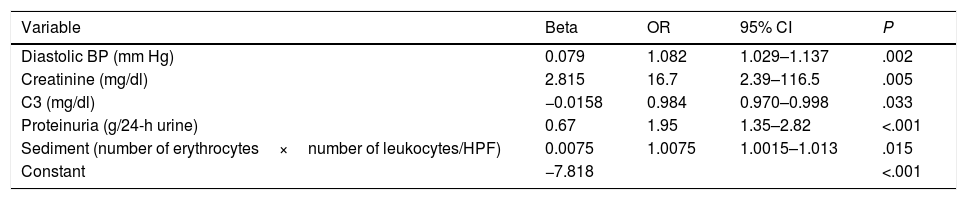
A significant interaction was detected between hematuria and leukocyturia (P=.007). Thus, in the final model the sediment variable (hematuria*leukocyturia) was included with significant improvement in discrimination (AUC=0.929; 95% CI=0.894–0.963) without calibration changes (P=.956). We also found a significant interaction between creatinine and proteinuria (P=.028). Nevertheless, its inclusion did not improve the model's performance nor did it show changes in the creatinine's effect at different proteinuria levels. Therefore, these two variables were left independent. The assumption of linearity and monotony was confirmed for all the variables included in the model. Table 4 shows the coefficients and their respective OR for the variables included in the final model.
Final Prediction Model of the Need for Aggressive Immunosuppression of Patients With Lupus Nephritis.
| Variable | Beta | OR | 95% CI | P |
|---|---|---|---|---|
| Diastolic BP (mm Hg) | 0.079 | 1.082 | 1.029–1.137 | .002 |
| Creatinine (mg/dl) | 2.815 | 16.7 | 2.39–116.5 | .005 |
| C3 (mg/dl) | −0.0158 | 0.984 | 0.970–0.998 | .033 |
| Proteinuria (g/24-h urine) | 0.67 | 1.95 | 1.35–2.82 | <.001 |
| Sediment (number of erythrocytes×number of leukocytes/HPF) | 0.0075 | 1.0075 | 1.0015–1.013 | .015 |
| Constant | −7.818 | <.001 |
Bootstrapping on 200 repetitions showed inconsistency in sediment (P=.052) and complement C3 (P=.062) coefficients. Despite the apparent instability of these variables, their importance and clinical significance as measures of general activity of SLE and renal inflammation, forced to consider them in external validation and in future studies of clinical use.
A spreadsheet calculator (supplementary appendix) was developed to estimate the probability of needing aggressive immunosuppression with cytostatics using a logistic regression equation with the variables included in the model:
where dbp=diastolic BP in mm Hg, Cr=creatinine in mg/dl, C3=complement C3 in mg/dl, Prot=proteinuria in 24h in mg, h=hematuria (maximum number of erythrocytes per high power field), l=leukocyturia (maximum number of leukocytes per high power field).As an exploratory approach, different cut-off points were sought for the needing of aggressive immunosuppression with high specificity or with high sensitivity to confirm or discard, respectively. Based on this equation, and considering as a positive cut-off point the probability of 0.8, an 81.3% sensitivity and 92.7% specificity with a positive likelihood ratio (LR)=11 was obtained. On the other hand, under a probability of 0.4 a 95.6% sensitivity and 46.4% specificity with a negative LR=0.09 was obtained. On the whole cohort, 178 patients (80%) were classified as above 0.8 or under 0.4. In the gray zone between these two cut-off points, 45 of 223 patients (20%) might have to undergo kidney biopsy to decide their treatment.
DiscussionThe therapeutic options currently available for LN patients are limited and, in the real clinical scenario, the situation is limited to defining for each case whether it is necessary to add high doses of cytostatics medications to conventional treatment. Traditionally, clinicians have considered that an individual analysis of clinical and laboratory variables does not allow establishing which patients have forms of LN that require aggressive treatment. For the first time, in accordance with the literature reviewed, a clinical prediction model was developed for the needing of aggressive immunosuppression with cytostatics in SLE patients over 16 years of age and with nephritis with less than 6 months of evolution of the symptoms attributable to nephritis. One of the main advantages of the model is that it is exclusively based on easy to measure routine clinical and laboratory parameters in the study of every SLE patient. Furthermore, there is no verification bias because a biopsy is performed equally on everyone suspected of nephritis regardless if clinical and laboratory findings are slight or severe.
Our model involves the variables diastolic BP, creatinine, C3, proteinuria and sediment, a variable that combines hematuria and leukocyturia. Even though this is the first study attempting to develop a multivariate model, other authors have previously attempted to find out which variables relate with the same outcome considered here. Wakasugi et al. found that in silent-nephritis patients the high titers of anti-DNA antibodies and the very low levels of complement C3 are related to the finding of a form of class III or IV proliferative nephritis.15 Likewise, Mitjavila et al. reported that the high titers of anti-DNA and lower levels of hemolytic complement CH100 were highly suggestive of proliferative glomerulonephritis.13 In another recent study of clinical pathological correlation, it was found that patients with proliferative class IV LN more frequently presented hematuria, proteinuria, hypertension, increase of blood ureic nitrogen, anemia, hypoalbuminemia and positive anti-DNA antibodies.14 In an additional study, a univariate retrospective analysis was conducted to identify factors associated with proliferative LN and then it was evaluated the diagnostic performance of different mixed models combining some of these factors.16 The variables related to a proliferative form of nephritis were male gender, hematuria, proteinuria, hypoalbuminemia, and C3 and C4 hypocomplementemia, as well as high titers of anti-DNA antibodies. Despite several of these studies showed association between anti-DNA antibodies and proliferative nephritis, this variable was not included in our final model. Although it is possible that such variable does not add extra information to the “renal inflammation” showed by C3, proteinuria and sediment34; some type of residual confusion due to technical and operational limitations in the method of indirect immunofluorescence cannot be discarded.35,36 Other studies suggest poor clinical–histological correlation due to the presence of proliferative forms in patients with low proteinuria with or without hematuria, or the presence of nonproliferative forms in patients presenting nephrotic syndrome. These discrepancies with our results may be due to the univariate approach in their design or the particular selection of patients and populations included in each case.37–39
Our model is encompassed within the same logic of some groups’ proposal to use the biopsy more conservatively, with an initial treatment guided by clinical presentation patterns and reserve biopsies for those cases in which it is not possible to identify a clear clinical presentation pattern, the patient is not improving with standard treatment or when there is a suspicion that there is another cause of nephritis.8,9 Some studies compared treatment based on diagnostic of proliferative LN using clinical and laboratory information with patients whose treatment was based on the result of a kidney biopsy, without differences in prognosis or the preservation of kidney function.17,40,41 Among them, a Mexican40 and a Brazilian study,41 close to our study population, suggested that the clinical and laboratory information may be enough to diagnose and treat LN, without resorting to a kidney biopsy.
If externally validated, the proposed model would be useful to start at the bedside a suitable treatment for lupus nephritis. For instance, it would work in geographical zones or times of the year when there is no availability of a kidney biopsy, in anticoagulated patients or in those who reject to undergo an invasive procedure. In general, any patient could have the choice to use the equation we have developed to estimate the probability of requiring intensive immunosuppression or undergoing kidney biopsy.
Some limitations of this study include its retrospective design, which favors missing data and reduces sample size. Information regarding race, serum albumin level or the presence of cellular casts in urinary sediment was not always available. Hence, the reduced number of outcomes and of independent candidate variables poses the risk of overfitting and of a very optimistic performance of the model. Additionally, the study only included patients from two third-level hospitals, those that usually perform kidney biopsies. This potential selection bias could overestimate the predictive capacity of the model, because of a greater frequency of severe forms of nephritis that need aggressive immunosuppression.
Pediatric population, specifically those younger than 16 years, was also excluded. In order to develop a similar model for those patients it would be necessary to adjust many of the proposed variables as age, weight or body surface area, as well as much other information not collected in our study.
Finally, despite the result of the kidney biopsy is the most practical and immediate outcome, its reliability is far from being optimal to be considered as a perfect gold standard.42–44 Perhaps a clinical study for this research question should predict the final clinical decision along with patients’ long-term outcome.
We believe that in recent-onset LN patients, the decision to use or not to use intensive therapy with cytostatics could be made based on our multivariate prediction model as an alternative to a kidney biopsy. This predictive model of diagnosis of proliferative nephritis is important because it could guide physicians at the time of making therapeutic decisions for a patient with lupus and renal involvement. Implementation of our model could reduce costs, complications and delays caused by renal biopsies usually requested to star treatment of these patients. Additional research is necessary before extrapolating our model to other populations. Furthermore, it is necessary to conduct an external validation and then a study on its clinical impact to sustain its generalized use.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis study was conducted to earn a degree of Master's in Clinical Sciences at Universidad de Antioquia. Universidad de Antioquia 2013–2014 Sustainability strategy, Clinical Epidemiology Academic Group – GRAEPIC (In Spanish, Grupo Académico de Epidemiología Clínica).
Conflict of InterestsThe authors declare that there is no conflict of interests.