The course and long-term outcome of pure membranous lupus nephritis (MLN) are little understood. The aims of this study are to evaluate the clinical features, course, outcome and prognostic indicators in pure MLN and to determine the impact of ethnicity and the type of health insurance on the course and prognosis of pure MLN.
MethodsWe conducted a retrospective review of medical records of 150 patients with pure MLN from Spain and the USA.
ResultsMean age was 34.2±12.5 and 80% were women. Sixty-eight percent of patients had nephrotic syndrome at diagnosis. The average serum creatinine was 0.98±0.78mg/dl. Six percent of patients died and 5.3% developed end-stage renal disease (ESRD). ESRD was predicted by male sex, hypertension, dyslipidemia, high basal 24h-proteinuria, high basal serum creatinine and a low basal creatinine clearance. Age, cardiac insufficiency, peripheral artheriopathy, hemodialysis and not having received mycophenolate mofetil or antimalarials for MLN predicted death.
ConclusionsPure MLN frequently presents with nephrotic syndrome, high proteinuria and normal serum creatinine. Its prognosis is favourable in maintaining renal function although proteinuria usually persists over time. Baseline cardiovascular disease and not having a health insurance are related with poor prognosis.
Los conocimientos sobre el curso y el desenlace a largo plazo de la nefritis lúpica membranosa (NLM) pura son todavía escasos. El objetivo de este estudio es evaluar las características clínicas, curso, desenlace e indicadores pronósticos de la NLM y determinar el impacto de la etnicidad y tipo de cobertura sanitaria en el curso y pronóstico de la NLM.
MétodosSe realizó una revisión retrospectiva de las historias de 150 pacientes con NLM de España y Estados Unidos.
ResultadosLa edad media fue 34,2±12,5 y el 80% eran mujeres. El 68% de los pacientes tenían síndrome nefrótico al diagnóstico. La creatinina sérica media fue 0,98±0,78mg/dl. El 6% de los pacientes fallecieron y el 5,3% desarrollaron insuficiencia renal terminal (IRT). El sexo masculino, la hipertensión, la dislipemia, la alta proteinuria basal, la alta creatininemia y un aclaramiento de creatinina reducido predijeron el desarrollo de IRT. La edad, la insuficiencia cardíaca, la arteriopatía periférica, la hemodiálisis y el no haber recibido micofenolato de mofetilo o antimaláricos predijeron el fallecimiento.
ConclusionesLa NLM pura suele debutar con síndrome nefrótico, alta proteinuria y creatininemia normal. Su pronóstico es favourable en términos de mantenimiento de la función renal aunque la proteinuria habitualmente persiste durante el seguimiento. La enfermedad cardiovascular basal y no tener cobertura sanitaria se relacionan con mal pronóstico.
Over 50% of patients with systemic lupus erythematosus (SLE) have clinically significant renal involvement during the course of their disease. Approximately 8–20% of these are found on renal biopsy to have membranous lupus nephropathy (MLN).1,2 Pure MLN has distinct histologic features compared to proliferative lupus nephritis.3 This difference is reflected in the clinical presentations of proliferative and membranous lupus nephritis, with the former being dominated by a nephritic process whereas MLN is characterised predominantly by nephrotic syndrome.1,4,5
Few studies have addressed the course and outcome of MLN directly,4,6–14 and their results are contrasting. Most of our knowledge is derived from extrapolations of data of idiopathic membranous nephritis and/or proliferative lupus nephritis. Research on MLN frequency, natural history, prognosis and treatment was further limited due to a lack of uniform definition over the past several decades, making it difficult to get consistent information from the published literature about MLN.1
An Italian study15 has reported the outcome in 103 patients with MLN, but a third of them had mixed forms. More recently, Mejía-Vilet et al.13 have reported the results of a cohort of 60 Hispanic and Mexican-mestizo patients with pure MLN suggesting an impact of ethnicity on the response to different immunosuppressants (azathioprine, cyclophosphamide or mycophenolate).
Large cohorts of patients with pure MLN are lacking, and to date the influence of ethnicity and social conditions on pure MLN outcome has rarely been studied.
The aims of the present study are to evaluate the clinical features, course, outcome and prognostic indicators in pure MLN and to explore the association of ethnicity, socio-economic level, country of residence and the type of health insurance with the course and prognosis of pure MLN.
MethodsPatientsThis multicentre, retrospective clinicopathologic study evaluated 150 patients with biopsy-proven Class Va pure MLN (modified WHO classification) who underwent renal biopsies between 1978 and 2011 in 24 hospitals in Spain and 1 in the United States. With the aim of gathering a large number of pure MLN patients and taking into account its low prevalence, we chose to include patients diagnosed in a broad temporal range and from different geographical areas. The registers of renal biopsies of each hospital were used to identify eligible patients. Patients were included if they met at least 4 American College of Rheumatology criteria for SLE16,17 with evidence of kidney involvement and biopsy-proven pure MLN.18 Mixed forms of MLN were excluded. The start of the study for each patient was the day of the renal biopsy diagnosed MLN. The end of follow-up for each patient was the day of the last visit available in the medical records. If the patient had been rebiopsied and the histological class had changed, he/she was then excluded from the analysis. The end of follow-up was the day of the last visit available in the clinical records by July 2011.
Ethical approval was obtained from the Hospital Universitario Puerta de Hierro Majadahonda Ethics Committee in Spain and the Hospital for Joint Diseases Ethics Committee in the USA.
Baseline AssessmentBaseline clinical data included age, sex, ethnicity, weight, body mass index, smoking status (current, ex, never), education (none, primary school, high school, university) and type of health insurance (none, public, private). The presence of a diagnosis of diabetes mellitus, hypertension, infection by Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), Human Immunodeficiency Virus (HIV) or tuberculosis, liver disease, ischaemic cardiopathy, cardiac failure, cerebrovascular arteriopathy, peripheral arteriopathy, neoplasm, osteoporosis and depression in the clinical records, either previously or at the time of MLN diagnosis, was also recorded. Moreover, all SLE clinical manifestations, classified by organ and/or system, at the time of MLN diagnosis were also recorded as well as the Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI). Information regarding the treatments received for SLE or comorbidities was registered.
The following laboratory values at the time of biopsy were analysed: complete blood count, erythrosedimentation rate (ESR), C-reactive protein (CRP), serum creatinine, serum albumin, serum cholesterol and triglycerides, 24-h proteinuria, urine sediment, serum complement (C3 and C4), the value of serum antinuclear antibody (ANA), the presence and level of serum antibodies to double stranded DNA (dsDNA), the presence of serum antibodies to non-DNA nuclear and cytosolic antigens (Sm, nRNP, SSA, SSB), and of antiphospholipid (aPL) antibodies (lupus anticoagulant and/or anticardiolipine antibody and/or anti-β2 glycoprotein I antibody). Creatinine clearance was calculated using the Cockcroft and Gault formula.
We recorded the presence of nephrotic syndrome at MLN diagnosis defined as presentation with urine protein excretion of 3g/24h or greater, serum hypoalbuminemia and lower limbs oedema simultaneously.
At the end of the follow-up, the same parameters were tested and recorded along with the date of the test. Treatment prescribed just after the diagnosis of MLN was recorded including corticosteroids, antimalarials, immunosuppressants (cyclophosphamide, azathioprine, mofetil mycophenolate, methotrexate and rituximab among other), adjuvant therapy (antihypertensive, hypolipidemic, antiaggregant and anticoagulant drugs), plasmapheresis, dialysis and renal transplant.
Study OutcomesMajor end points were either death or end-stage renal disease (ESRD) requiring chronic renal replacement therapy (dialysis or transplant). Secondary end points included 24h-proteinuria under 0.5g, 24h-proteinuria under 1g, doubling of serum creatinine, renal failure defined as serum creatinine ≥1.2mg/dL, and hypertension defined as systolic blood pressure (BP) greater than 140mm Hg and/or diastolic BP greater than 90mm Hg.
Statistical AnalysisIn order to know the general characteristics of both populations (Spanish and North American), a descriptive statistical analysis with the initial variables was undertaken. Measures of central tendency and dispersion such as mean, median, standard deviation and extreme values were used as descriptive statistics. Equality of distributions was investigated using the Kolmogorov–Smirnov test. Continuous data are presented as mean±standard deviation. Student's t-test or Mann–Whitney U-test were used for comparison of continuous variables and the Chi-square test for categorical variables.
Multiple logistic regression analysis was conducted to estimate the factors associated with the different outcomes, adjusting by hospital, ethnicity, education level and type of health coverage. Variables included in the model were selected using a backward elimination for variables with a P>.05. Crude odds ratios (ORs) with 95% confidence intervals (CIs) and P values based on two-sided X2 tests (continuous variables) or Fisher exact tests (categorical variables) were calculated for all possible predictive factors. All the comparisons were with bilateral contrasts with an established level of statistic significance in values ≤0.05.
Statistical Package for Social Sciences (SPSS) v14.0 software for Windows was used for all statistical analysis.
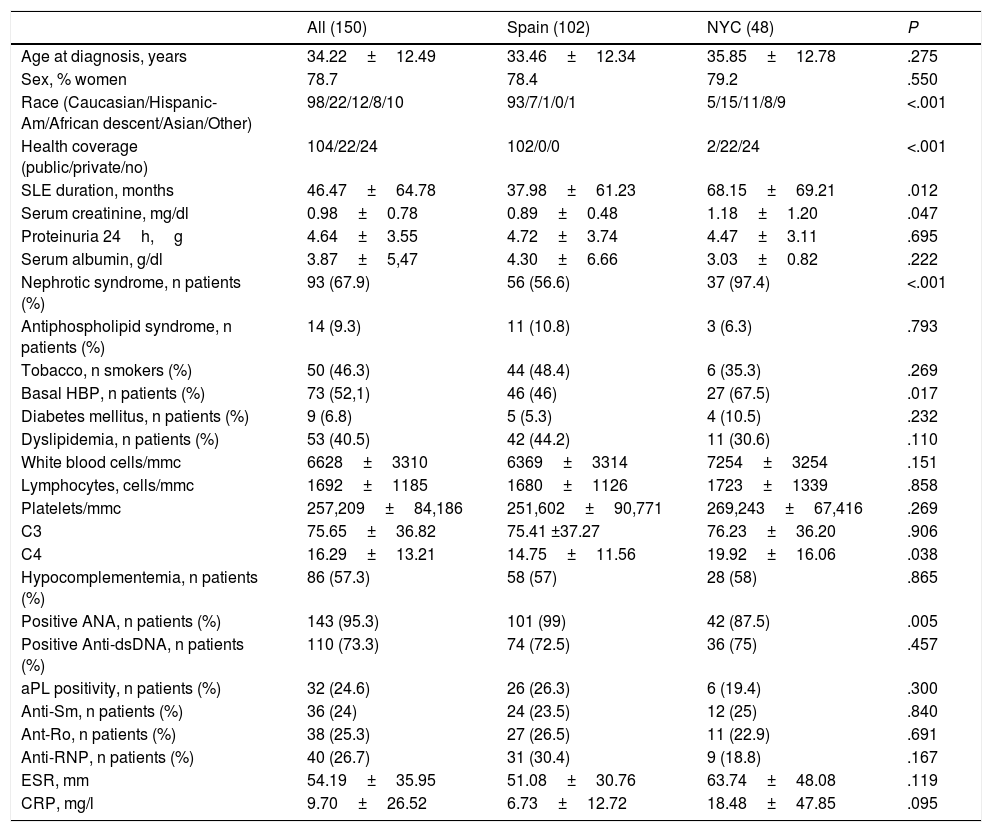
ResultsComparison Between Both CohortsOf the 150 patients included in the analysis, 48 were followed-up in the Hospital for Joint Diseases of New York and 102 in the rheumatology departments of 24 different hospitals in Spain. The clinical characteristics at MLN presentation, administered treatments at any point during the course of the disease and outcome of both cohorts are displayed in Tables 1–3. Statistically significant differences between both cohorts were found regarding ethnicity distribution, type of health coverage, SLE duration at MLN diagnosis (longer for North Americans) and presentation with nephrotic syndrome and high blood pressure (both more frequent among North Americans). Of the laboratory values, Spaniards had a lower C4 and a higher percentage of ANA antibodies positivity at MLN diagnosis. SELENA-SLEDAI at the point of MLN diagnosis was only calculated for the Spanish cohort (12.5±5.7), as was creatinine clearance (97.2±44.7ml/min) since not all the individual components of these indexes were available for the North-American cohort.
Baseline Characteristics of the Included Patients.
| All (150) | Spain (102) | NYC (48) | P | |
|---|---|---|---|---|
| Age at diagnosis, years | 34.22±12.49 | 33.46±12.34 | 35.85±12.78 | .275 |
| Sex, % women | 78.7 | 78.4 | 79.2 | .550 |
| Race (Caucasian/Hispanic-Am/African descent/Asian/Other) | 98/22/12/8/10 | 93/7/1/0/1 | 5/15/11/8/9 | <.001 |
| Health coverage (public/private/no) | 104/22/24 | 102/0/0 | 2/22/24 | <.001 |
| SLE duration, months | 46.47±64.78 | 37.98±61.23 | 68.15±69.21 | .012 |
| Serum creatinine, mg/dl | 0.98±0.78 | 0.89±0.48 | 1.18±1.20 | .047 |
| Proteinuria 24h,g | 4.64±3.55 | 4.72±3.74 | 4.47±3.11 | .695 |
| Serum albumin, g/dl | 3.87±5,47 | 4.30±6.66 | 3.03±0.82 | .222 |
| Nephrotic syndrome, n patients (%) | 93 (67.9) | 56 (56.6) | 37 (97.4) | <.001 |
| Antiphospholipid syndrome, n patients (%) | 14 (9.3) | 11 (10.8) | 3 (6.3) | .793 |
| Tobacco, n smokers (%) | 50 (46.3) | 44 (48.4) | 6 (35.3) | .269 |
| Basal HBP, n patients (%) | 73 (52,1) | 46 (46) | 27 (67.5) | .017 |
| Diabetes mellitus, n patients (%) | 9 (6.8) | 5 (5.3) | 4 (10.5) | .232 |
| Dyslipidemia, n patients (%) | 53 (40.5) | 42 (44.2) | 11 (30.6) | .110 |
| White blood cells/mmc | 6628±3310 | 6369±3314 | 7254±3254 | .151 |
| Lymphocytes, cells/mmc | 1692±1185 | 1680±1126 | 1723±1339 | .858 |
| Platelets/mmc | 257,209±84,186 | 251,602±90,771 | 269,243±67,416 | .269 |
| C3 | 75.65±36.82 | 75.41 ±37.27 | 76.23±36.20 | .906 |
| C4 | 16.29±13.21 | 14.75±11.56 | 19.92±16.06 | .038 |
| Hypocomplementemia, n patients (%) | 86 (57.3) | 58 (57) | 28 (58) | .865 |
| Positive ANA, n patients (%) | 143 (95.3) | 101 (99) | 42 (87.5) | .005 |
| Positive Anti-dsDNA, n patients (%) | 110 (73.3) | 74 (72.5) | 36 (75) | .457 |
| aPL positivity, n patients (%) | 32 (24.6) | 26 (26.3) | 6 (19.4) | .300 |
| Anti-Sm, n patients (%) | 36 (24) | 24 (23.5) | 12 (25) | .840 |
| Ant-Ro, n patients (%) | 38 (25.3) | 27 (26.5) | 11 (22.9) | .691 |
| Anti-RNP, n patients (%) | 40 (26.7) | 31 (30.4) | 9 (18.8) | .167 |
| ESR, mm | 54.19±35.95 | 51.08±30.76 | 63.74±48.08 | .119 |
| CRP, mg/l | 9.70±26.52 | 6.73±12.72 | 18.48±47.85 | .095 |
ACEI, angiotensin converting enzyme inhibitor; aPL, antiphospholipidic; ARB, angiotensin receptor blockers; CRP, C reactive protein; ESR, erythrosedimentation rate; ESRD, end-stage renal disease; HBP, high blood pressure; MMF, mophetil mycophenolate; SLE, systemic lupus erythematosus.
Treatment Given for Membranous Lupus Nephritis.
| Drug | All (150) | Spain (102) | NYC (48) | P |
|---|---|---|---|---|
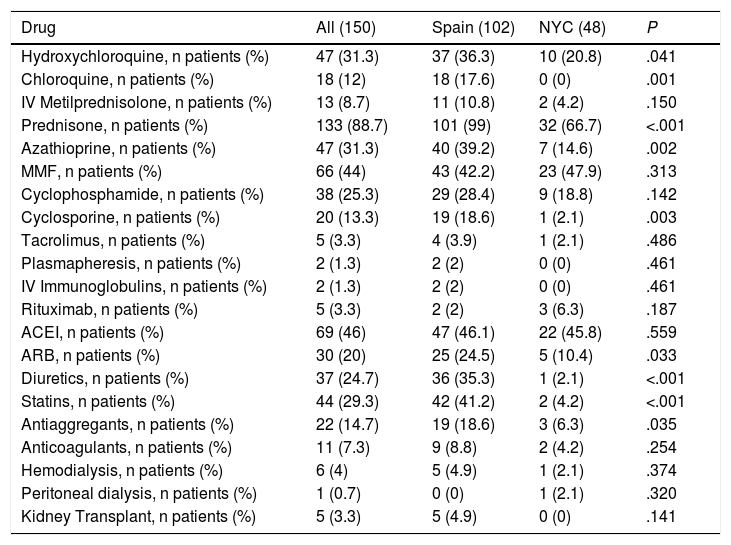
| Hydroxychloroquine, n patients (%) | 47 (31.3) | 37 (36.3) | 10 (20.8) | .041 |
| Chloroquine, n patients (%) | 18 (12) | 18 (17.6) | 0 (0) | .001 |
| IV Metilprednisolone, n patients (%) | 13 (8.7) | 11 (10.8) | 2 (4.2) | .150 |
| Prednisone, n patients (%) | 133 (88.7) | 101 (99) | 32 (66.7) | <.001 |
| Azathioprine, n patients (%) | 47 (31.3) | 40 (39.2) | 7 (14.6) | .002 |
| MMF, n patients (%) | 66 (44) | 43 (42.2) | 23 (47.9) | .313 |
| Cyclophosphamide, n patients (%) | 38 (25.3) | 29 (28.4) | 9 (18.8) | .142 |
| Cyclosporine, n patients (%) | 20 (13.3) | 19 (18.6) | 1 (2.1) | .003 |
| Tacrolimus, n patients (%) | 5 (3.3) | 4 (3.9) | 1 (2.1) | .486 |
| Plasmapheresis, n patients (%) | 2 (1.3) | 2 (2) | 0 (0) | .461 |
| IV Immunoglobulins, n patients (%) | 2 (1.3) | 2 (2) | 0 (0) | .461 |
| Rituximab, n patients (%) | 5 (3.3) | 2 (2) | 3 (6.3) | .187 |
| ACEI, n patients (%) | 69 (46) | 47 (46.1) | 22 (45.8) | .559 |
| ARB, n patients (%) | 30 (20) | 25 (24.5) | 5 (10.4) | .033 |
| Diuretics, n patients (%) | 37 (24.7) | 36 (35.3) | 1 (2.1) | <.001 |
| Statins, n patients (%) | 44 (29.3) | 42 (41.2) | 2 (4.2) | <.001 |
| Antiaggregants, n patients (%) | 22 (14.7) | 19 (18.6) | 3 (6.3) | .035 |
| Anticoagulants, n patients (%) | 11 (7.3) | 9 (8.8) | 2 (4.2) | .254 |
| Hemodialysis, n patients (%) | 6 (4) | 5 (4.9) | 1 (2.1) | .374 |
| Peritoneal dialysis, n patients (%) | 1 (0.7) | 0 (0) | 1 (2.1) | .320 |
| Kidney Transplant, n patients (%) | 5 (3.3) | 5 (4.9) | 0 (0) | .141 |
ACEI, angiotensin converting enzyme inhibitor; aPL, antiphospholipidic; ARB, angiotensin receptor blockers; CRP, C reactive protein; ESR, erythrosedimentation rate; ESRD, end-stage renal disease; HBP, high blood pressure; MMF, mophetil mycophenolate; SLE, systemic lupus erythematosus.
Outcome of MLN in the Entire Cohort.
| All (150) | Spain (102) | NYC (48) | P | |
|---|---|---|---|---|
| Mean follow-up, months | 91.18±89.42 | 117.73±95.03 | 34.77±34.16 | <.0001 |
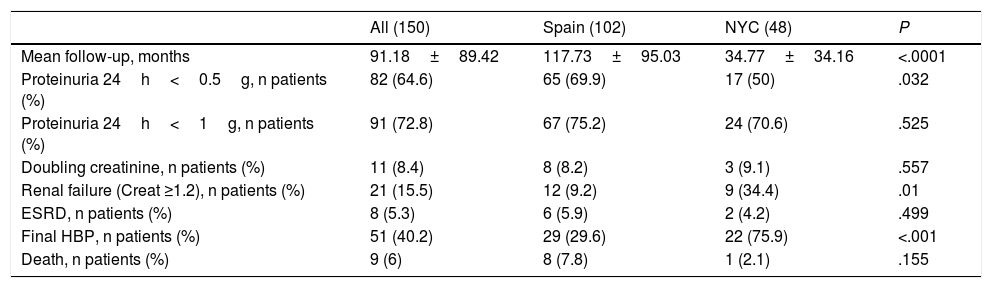
| Proteinuria 24h<0.5g, n patients (%) | 82 (64.6) | 65 (69.9) | 17 (50) | .032 |
| Proteinuria 24h<1g, n patients (%) | 91 (72.8) | 67 (75.2) | 24 (70.6) | .525 |
| Doubling creatinine, n patients (%) | 11 (8.4) | 8 (8.2) | 3 (9.1) | .557 |
| Renal failure (Creat ≥1.2), n patients (%) | 21 (15.5) | 12 (9.2) | 9 (34.4) | .01 |
| ESRD, n patients (%) | 8 (5.3) | 6 (5.9) | 2 (4.2) | .499 |
| Final HBP, n patients (%) | 51 (40.2) | 29 (29.6) | 22 (75.9) | <.001 |
| Death, n patients (%) | 9 (6) | 8 (7.8) | 1 (2.1) | .155 |
ACEI, angiotensin converting enzyme inhibitor; aPL, antiphospholipidic; ARB, angiotensin receptor blockers; CRP, C reactive protein; ESR, erythrosedimentation rate; ESRD, end-stage renal disease; HBP, high blood pressure; MMF, mophetil mycophenolate; SLE, systemic lupus erythematosus.
In terms of treatment received for MLN, oral steroids, antimalarials, immunosuppressive therapy with azathioprine (AZA) and cyclosporine (CsA), and adjuvant therapy with diuretics, antihypertensive and antilipidemic drugs were more often used in Spanish patients. As a whole, 17% (26 patients) of the cohort were treated only with steroids, 25% (37 patients) received steroids and only one immunosuppressant drug, and 70 patients (47%) received more than one immunosuppressant plus steroids. Seventeen patients did not receive oral steroids for MLN.
The overall group was followed up for more than 7.5 years. Mean follow-up was longer for Spaniards (117 months vs 34 months for USA patients). A significantly higher percentage of Spanish patients achieved a final 24h-proteinuria under 0.5g, meaning a better prognosis. Furthermore, a higher percentage of North-American patients had hypertension and renal failure at the end of follow-up. There were no significant differences between the both groups in the percentage of patients who developed end-stage renal disease or died.
Predictors of Renal OutcomeWe looked for predictors of the different outcomes in the entire group. In terms of comorbidities, several cardiovascular conditions including ischaemic cardiopathy (P=.001), cardiac failure (P=.013), cerebrovascular (P=.012) and peripheral (P=.012) vascular disease, were significantly associated with death. Patients who died were older at MLN diagnosis (P=.006). Hemodialysis (P=.03) and not having received mofetil mycophenolate (P=.039) or hydroxichloroquine (P=.03) for MLN predicted death.
ESRD was associated with male sex (P=.011), basal hypertension (P=.005), dyslipidemia (P=.017), high basal 24 h-proteinuria (7.57 vs 4.47g; P=.016), high basal serum creatinine (2.76 vs 0.91mg/dl; P<.001), nephrotic syndrome (P=.041) and a low creatinine clearance (37 vs 102ml/min; P<.001).
Patients with final doubled creatinine had a higher initial serum creatinine (1.92 vs 0.86mg/dl; P<.001) and a lower creatinine clearance (59 vs 102ml/min; P=.016). Male sex (P<.001), basal hypertension (P<.001) and some cardiovascular conditions such as ischaemic cardiopathy (P=.02), chronic cardiopathy (P=.018) and peripheral vascular disease (P=.039) were also associated with doubling basal serum creatinine.
Lastly, female sex (P=.017), a low basal serum creatinine (0.85 vs 1.39mg/dl; P=.001) and previous treatment with AZA (P=.03) were predictors of a final proteinuria <1g. Nevertheless, North-American patients (P=.042), patients without a Health insurance (P=.031), those positive to anti-dsDNA (P=.047) or aPL (P=.026) antibodies and those who had suffered a previous venous thrombosis (P=.036) had less chances of achieving a final proteinuria <0.5g.
Multivariable analysis showed that patients who finally achieved a 24h proteinuria <0.5g, had public health insurance (odds ratio [OR] 2.2; 95% confidence interval [CI], 0.72–6.74; P=.165), a lower basal serum creatinine (OR 0.57; 95% CI, 0.27–1.17; P=.126) and had received AZA for the treatment of MLN (OR 2.2, 95% CI, 0.91–5.3; P=.011). A predictive model with these three variables would have an accuracy of 0.67 and a negative likelihood ratio (LR) for achieving a final proteinuria <0.5g of 0.33.
Patients without leukopenia (OR 0.31; 95% CI, 0.11–0.87; P=.028) and with high serum creatinine (OR 0.26; 95% CI, 0.09–0.81; P=.020) at the time of MLN diagnosis had less chances of achieving a final 24h proteinuria <1g. The model based in these two variables could predict a final proteinuria <1g with an accuracy of 0.77, a positive LR 2.1 and negative LR 0.34.
And men (OR 6.53; 95% CI, 1.33–32.06; P=.021) with a higher basal serum creatinine (OR 3.23; 95% CI 0.97–10.69; P=.05) were more likely to have doubling basal serum creatinine at the end of the follow-up. This could be predicted with an accuracy of 0.8, positive LR 13.5 and negative LR 0.73.
Multivariable analysis was not performed for death and ESRD due to the scarce number of cases that achieved these outcomes in our cohort.
DiscussionIn this study, we retrospectively analysed the baseline and long-term follow-up characteristics of a cohort of 150 patients with pure MLN. Unlike many other studies, we excluded patients with mixed forms of membranous nephritis. There are only a few studies concerning pure MLN, and the conclusions about the presentation and natural course are unclear. This is possibly a consequence of the difficulties to identify histologically pure forms of MLN according to the different classifications and also of the limitations for the follow-up given that an optimal treatment for this type of lupus nephritis has not been established yet. Making inferences about pure MLN from cohorts that include different subtypes of class V lupus nephritis can be deceptive.10,19,20 To the best of our knowledge, this is the largest cohort on pure membranous lupus nephritis to be reported in the literature. It is also the first to compare two large groups of patients with MLN from different sociocultural settings.
It is noteworthy the high percentage of patients with positive anti-dsDNA antibodies in our series (73.3% of patients). MLN may be present with many, few or no other clinical or serologic manifestation of SLE.21,22 Anti-dsDNA antibodies are highly specific of SLE and their high prevalence in our cohort supports these were confirmed SLE diagnoses. This high percentage of anti-dsDNA positivity is similar to other series.13
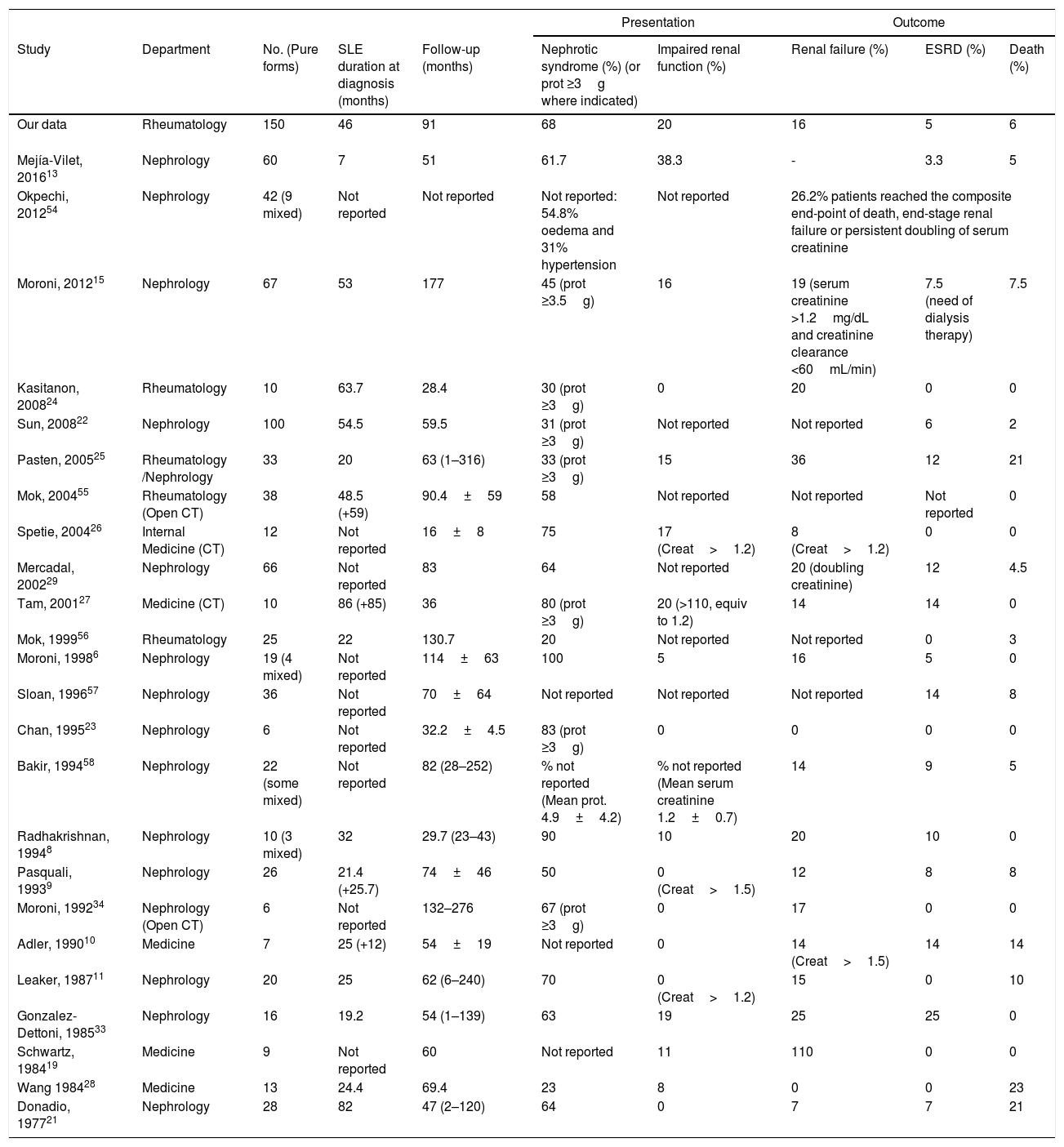
Patients’ characteristics at MLN presentation in our cohort are in agreement with those reported by other authors (Table 4). As in our cohort, MLN usually presents in women in their fourth decade of life and after a mean of four years after SLE diagnosis. Renal function is usually preserved at the beginning; impairment has been reported in 0–20% across different cohorts.6,8–11,19,20,23–28 Nephrotic proteinuria is found at diagnosis in a variable proportion of patients, and it usually evolves favourably with low percentages of death and/or ESRD at follow-up.13,22,24,27,29 The percentage of poor outcomes does not seem to vary with the length of the follow-up, since the renal survival is similar independently of the follow-up of the cohort. What seems to adversely influence the outcome is the presence and persistence of a nephrotic syndrome and having mixed proliferative lesions.4–6,8,9,30 In very old and short-term studies18,31 MLN was felt to exhibit an indolent and relatively benign course. However, subsequent long-term series have shown contrasting data about the prognosis of this disorder. Baldwin et al.32 found 71% of their 24 MLN patients to be persistently nephrotic; 25% progressed to renal failure over a period of 6 years. González-Dettoni et al.33 observed one the highest percentages of ESRD amongst published cohorts with 25% of their 16 patients after a 4.5 years follow-up. In contrast, ours and other cohorts13,18,19,21 have found a lower rate of progression to ESRD (Table 4). Moreover, a number of recent cohorts have even reported no patients progressing to ESRD.11,23,24,26,34 It is likely that different patient populations with varying epidemiological features such as ethnicity and socioeconomic level, different degrees of proteinuria and renal insufficiency, differing histologic features of MLN and diverse treatment protocols may account for these divergent results. Establishing these differences was precisely one of the major points of our comparison study between two international cohorts.
Series of Membranous Lupus Nephritis.
| Presentation | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Department | No. (Pure forms) | SLE duration at diagnosis (months) | Follow-up (months) | Nephrotic syndrome (%) (or prot ≥3g where indicated) | Impaired renal function (%) | Renal failure (%) | ESRD (%) | Death (%) |
| Our data | Rheumatology | 150 | 46 | 91 | 68 | 20 | 16 | 5 | 6 |
| Mejía-Vilet, 201613 | Nephrology | 60 | 7 | 51 | 61.7 | 38.3 | - | 3.3 | 5 |
| Okpechi, 201254 | Nephrology | 42 (9 mixed) | Not reported | Not reported | Not reported: 54.8% oedema and 31% hypertension | Not reported | 26.2% patients reached the composite end-point of death, end-stage renal failure or persistent doubling of serum creatinine | ||
| Moroni, 201215 | Nephrology | 67 | 53 | 177 | 45 (prot ≥3.5g) | 16 | 19 (serum creatinine >1.2mg/dL and creatinine clearance <60mL/min) | 7.5 (need of dialysis therapy) | 7.5 |
| Kasitanon, 200824 | Rheumatology | 10 | 63.7 | 28.4 | 30 (prot ≥3g) | 0 | 20 | 0 | 0 |
| Sun, 200822 | Nephrology | 100 | 54.5 | 59.5 | 31 (prot ≥3g) | Not reported | Not reported | 6 | 2 |
| Pasten, 200525 | Rheumatology /Nephrology | 33 | 20 | 63 (1–316) | 33 (prot ≥3g) | 15 | 36 | 12 | 21 |
| Mok, 200455 | Rheumatology (Open CT) | 38 | 48.5 (+59) | 90.4±59 | 58 | Not reported | Not reported | Not reported | 0 |
| Spetie, 200426 | Internal Medicine (CT) | 12 | Not reported | 16±8 | 75 | 17 (Creat>1.2) | 8 (Creat>1.2) | 0 | 0 |
| Mercadal, 200229 | Nephrology | 66 | Not reported | 83 | 64 | Not reported | 20 (doubling creatinine) | 12 | 4.5 |
| Tam, 200127 | Medicine (CT) | 10 | 86 (+85) | 36 | 80 (prot ≥3g) | 20 (>110, equiv to 1.2) | 14 | 14 | 0 |
| Mok, 199956 | Rheumatology | 25 | 22 | 130.7 | 20 | Not reported | Not reported | 0 | 3 |
| Moroni, 19986 | Nephrology | 19 (4 mixed) | Not reported | 114±63 | 100 | 5 | 16 | 5 | 0 |
| Sloan, 199657 | Nephrology | 36 | Not reported | 70±64 | Not reported | Not reported | Not reported | 14 | 8 |
| Chan, 199523 | Nephrology | 6 | Not reported | 32.2±4.5 | 83 (prot ≥3g) | 0 | 0 | 0 | 0 |
| Bakir, 199458 | Nephrology | 22 (some mixed) | Not reported | 82 (28–252) | % not reported (Mean prot. 4.9±4.2) | % not reported (Mean serum creatinine 1.2±0.7) | 14 | 9 | 5 |
| Radhakrishnan, 19948 | Nephrology | 10 (3 mixed) | 32 | 29.7 (23–43) | 90 | 10 | 20 | 10 | 0 |
| Pasquali, 19939 | Nephrology | 26 | 21.4 (+25.7) | 74±46 | 50 | 0 (Creat>1.5) | 12 | 8 | 8 |
| Moroni, 199234 | Nephrology (Open CT) | 6 | Not reported | 132–276 | 67 (prot ≥3g) | 0 | 17 | 0 | 0 |
| Adler, 199010 | Medicine | 7 | 25 (+12) | 54±19 | Not reported | 0 | 14 (Creat>1.5) | 14 | 14 |
| Leaker, 198711 | Nephrology | 20 | 25 | 62 (6–240) | 70 | 0 (Creat>1.2) | 15 | 0 | 10 |
| Gonzalez-Dettoni, 198533 | Nephrology | 16 | 19.2 | 54 (1–139) | 63 | 19 | 25 | 25 | 0 |
| Schwartz, 198419 | Medicine | 9 | Not reported | 60 | Not reported | 11 | 110 | 0 | 0 |
| Wang 198428 | Medicine | 13 | 24.4 | 69.4 | 23 | 8 | 0 | 0 | 23 |
| Donadio, 197721 | Nephrology | 28 | 82 | 47 (2–120) | 64 | 0 | 7 | 7 | 21 |
Creat, Creatinine (in mg/dl); CT, clinical trial; Prot., 24h-proteinuria (in g).
Some significant differences were found between our two cohorts. The American cohort had higher poor prognostic indicators than the Spanish cohort as a higher percentage of patients had nephrotic syndrome, high blood pressure and impaired renal function at disease presentation. SLE duration at MLN diagnosis was longer in the American cohort. This needs to be cautiously interpreted as it may reflect a more difficult access to health care rather than a milder disease in this population. The two cohorts were also quite different regarding the ethnic groups distribution. The North-American group included mainly Hispanic-American patients and patients of African descent whereas the majority of the Spanish patients were Caucasian. Our multivariable analysis did not show prognostic differences among the different ethnicities, probably due to the small number of patients of some of the ethnic groups in the sample. Nevertheless, it is well established that there are considerable racial differences in the prevalence, severity, and prognosis of lupus nephritis.35,36 Patients of African descent are more severely afflicted with SLE and have worse outcomes when compared with Caucasian patients.36,37 The reported prevalence of SLE is seven to eight times higher in Afro-Caribbeans, and two to four times higher in Asians than in Caucasians, while biopsy-proven lupus nephritis is 25 times more common in both these groups compared with Caucasians.38,39 It is also known that the response to therapy varies among different ethnicities.13 A higher rate of response to therapy has been described in Asiatic cohorts.40 On the other hand, Hispanics may have a higher response to mycophenolate than to cyclophosphamide as induction therapy and a lower rate of relapses when they receive mycophenolate as a maintenance treatment as compared with azathioprine.41–43
In addition to ethnicity, low socioeconomic status has also been associated with progression of SLE, mainly through increased cardiovascular risk.44 If we consider the type of health insurance as a proxy of the socioeconomic status for the North-American cohort, it could explain why this cohort had a more severe disease at presentation and a poorer prognosis. Nevertheless, due to the characteristics of the health system in Spain, which has universal health coverage, the type of health insurance cannot be taken as indicative of socioeconomic status for this population. The entire Spanish cohort had public health insurance whereas only 2 patients from the North-American cohort had this type of health insurance. Furthermore, half the North-American cohort had no health insurance at all. This fact may have delayed the diagnosis of MLN in USA patients and also limited the therapeutic possibilities influencing the control of the disease. We found significant differences in the use of oral steroids and several immunosuppressants in favour of the Spanish cohort. Although there is no standard immunosuppressive regimen for MLN, it is known that the addition of immunosuppressive drugs in combination with steroids may improve renal survival.45 The wide variation of treatment combinations in our cohort did not allow us to reach any conclusion about the optimal regimen, however our data suggests that an aggressive therapeutic approach influences the course of the apparently benign MLN. Patients from the Spanish cohort, who more often received immunosuppressive therapy, had a better prognosis, reflected by a higher percentage of patients achieving a 24h-proteinuria under 0.5g at the end of follow-up and lower percentage of patients developing high blood pressure. In particular, there was a notable difference in the use of cyclophosphamide (28.4% of patients in the Spanish cohort vs 18.8% in the American cohort) and azathioprine (39.2% vs 14.6%) between the two cohorts. It is likely that doctors in Spain prescribed immunosuppressants more frequently trying to avoid poor outcomes given that these treatments were easily available. Another explanation can be that these drugs were given to patients who either had relapses after treatment with only corticosteroids or were steroid-dependent.
Significant differences were also found regarding the use of antihypertensives, diuretics, statins and antiaggregants between both groups since they were more commonly used to treat patients in the Spanish cohort. Patients with MLN are at increased risk not only for ESRD but also cardiovascular complications45,46 and adjuvant therapy has an impact on the MLN prognosis.47 In fact, in our study, not only ESRD but also mortality was predicted mainly by cardiovascular comorbidities such as cardiac insufficiency, ischaemic cardiopathy and peripheral arteriopathy. Over the last few decades, with the decrease of early mortality due to uncontrolled disease, cardiovascular complications have emerged as important causes for late mortality.48 Since vascular complications are also accelerated in patients with renal failure, the risk of cardiovascular complications is compounded in patients with ESRD and SLE.49,50 Therefore, adjuvant therapy can have a positive influence on prognosis in patients with lupus nephritis.
We found significant differences in the time of follow-up. Spanish patients were followed three times longer than the USA cohort. Despite this notable difference, overall, the outcome was better for the Spanish cohort. It is possible that the USA cohort would have had even a worse prognosis if having been followed up for almost 10 years as the Spanish one. Nevertheless, there is no evidence that the length of follow-up conditions a poorer prognosis as presenting with nephrotic syndrome or having mixed lesions do.6,8,9
High basal proteinuria, as well as a high serum creatinine and a low creatinine clearance were all predictors of ESRD. This means that patients with a more severe basal nephritis had more chances of losing their renal function at follow-up. In the multivariable analysis, we only identified a low baseline serum creatinine and having health insurance as predictors of good prognosis in terms of achieving a 24h-proteinuria under 0.5g at the end of follow-up, but it is possible that in a larger sample factors such as ethnicity, cardiovascular conditions or nephrotic syndrome are identified as independent predictors. Nevertheless, no major prognostic indicators of MLN renal survival emerges from the literature. Mercadal et al.29 reported that a profound initial hypoalbuminaemia was a risk factor for ESRD and a sustained heavy proteinuria was a predictor of doubling of the serum creatinine. However other authors18,51 have found no correlation between the degree of proteinuria at the time of the renal biopsy and subsequent renal function deterioration among patients with MLN. Austin et al.52 identified proteinuria higher than 5g/d and treatment with prednisone alone as associated with a decreased probability of remission. Substantial data has established the prognostic importance of proteinuria in various glomerular diseases including idiopathic membranous nephropathy22,53 but it seems that in membranous lupus nephritis data are still contrasting.
Our study has several limitations. Although the Spanish MLN patients are well represented in our sample since they were followed up at 24 different participating centres, all the patients from the USA attended a single centre in New York. It is probable that this hospital does not represent the average standard of care in the USA. Thus, although our results lack of generalizability, the comparison between two international cohorts represents a strength as it brings up some determinants of pure MLN outcome not visible in more homogeneous cohorts. Since our aim was to make a description of MLN patients we did not record the date of events like death or ESRD and subsequently could not perform a survival analysis. The wide variety of therapies received by each patient prevented us from evaluating the effectiveness of each single immunosuppressant for pure MLN since the final outcome of the disease could not be associated with a specific drug. According to what has been previously reported6,8,9 we do not think that the difference in the mean follow-up between the two cohorts influenced the results; in any case, a longer follow-up for the USA cohort would have made the differences in prognosis between cohorts more obvious so it seems unlikely our findings can be explained by the different length of follow-up. A retrospective design is not the ideal methodology for prognostic studies. Ideally, a prospective study would identify true prognostic factors for MLN. Despite this, the large number of patients included in this cohort would enable probable prognostic factors to be studied in greater detail in future prospective cohorts. Furthermore, these preliminary results and our review of the literature draw attention to the influence of different types of health insurance and ethnicity in the prognosis of membranous lupus nephritis.
ConclusionMLN usually begins with nephrotic syndrome, high proteinuria and normal serum creatinine. Prognosis is favourable in terms of the maintenance of renal function, although proteinuria usually persists over time. Patient and renal survival are high in patients with pure types of MLN but severity at presentation predicts a poor long-term outcome in terms of ESRD. Other factors such as baseline cardiovascular disease and not having a health insurance are also related with poor prognosis.
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis study had no funding source or sponsor.
Ethics approval: Ethical approval for this study was obtained in February 2011 from the Hospital Universitario Puerta de Hierro Majadahonda of Madrid, Spain.
Conflicts of InterestThe authors declare no conflicts of interest.