Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA). We characterized efficacy and safety of tofacitinib in Mexican patients from RA Phase 3 and long-term extension (LTE) studies.
MethodsData from Mexican patients with RA and an inadequate response to disease-modifying antirheumatic drugs (DMARDs) were taken from four Phase 3 studies (pooled across studies) and one open-label LTE study of tofacitinib. Patients received tofacitinib 5 or 10mg twice daily, adalimumab (one Phase 3 study) or placebo (four Phase 3 studies) as monotherapy or in combination with conventional synthetic DMARDs. Efficacy up to Month 12 (Phase 3) and Month 36 (LTE) was assessed by American College of Rheumatology 20/50/70 response rates, Disease Activity Score (erythrocyte sedimentation rate), and Health Assessment Questionnaire-Disability Index. Safety, including incidence rates (IRs; patients with events/100 patient-years) for adverse events (AEs) of special interest, was assessed throughout the studies.
Results119 and 212 Mexican patients were included in the Phase 3 and LTE analyses, respectively. Tofacitinib-treated patients in Phase 3 had numerically greater improvements in efficacy responses versus placebo at Month 3. Efficacy was sustained in Phase 3 and LTE studies. IRs for AEs of special interest were similar to those with tofacitinib in the global and Latin American RA populations.
ConclusionsIn Mexican patients from the tofacitinib global RA program, tofacitinib efficacy was demonstrated up to Month 12 in Phase 3 studies and Month 36 in the LTE study, with a safety profile consistent with tofacitinib global population.
Tofacitinib es un inhibidor de la cinasa Janus para el tratamiento de la artritis reumatoide (AR). Se evaluaron la eficacia y la seguridad de tofacitinib en pacientes mexicanos a partir de los estudios fase 3 y de extensión a largo plazo (ELP) de AR.
MétodosDatos de pacientes mexicanos con AR y respuesta inadecuada a fármacos antirreumáticos modificadores de la enfermedad (FARME) fueron tomados de 4 estudios fase 3 y de un estudio abierto de ELP de tofacitinib. Los pacientes recibieron tofacitinib 5 o 10mg 2 veces al día, adalimumab (en un estudio fase 3) o placebo (en 4 estudios fase 3) como monoterapia o en combinación con FARME sintético convencional. Se evaluó la eficacia al mes 12 (fase 3) y al mes 36 (ELP) por medio de las tasas de respuesta del Colegio Americano de Reumatología 20/50/70, el puntaje de actividad de la enfermedad (DAS) 28-4, velocidad de sedimentación globular y el índice de discapacidad del cuestionario de evaluación de la salud (HAQ-DI). Se evaluó la seguridad a través de los estudios, incluyendo tasas de incidencia (IR; pacientes con evento/100 pacientes-año).
ResultadosCiento diecinueve y 212 pacientes mexicanos fueron incluidos en el análisis de los estudios fase 3 y de extensión a largo plazo, respectivamente. Pacientes tratados con tofacitinib en los estudios fase 3, numéricamente, tuvieron una mayor mejoría en las respuestas de eficacia en comparación con el placebo al mes 3. La eficacia fue sostenida en los estudios fase 3 y de extensión a largo plazo. Las tasas de incidencia de los eventos adversos de especial interés fueron similares a aquellas con tofacitinib en la población global y latinoamericana.
ConclusionesEn pacientes mexicanos del programa global de tofacitinib en AR, la eficacia de tofacitinib se demostró hasta el mes 12 en los estudios fase 3 y hasta el mes 36 en el estudio de extensión a largo plazo, con un perfil de seguridad consistente con el de la población global de los estudios de tofacitinib.
Rheumatoid arthritis (RA) is a chronic, debilitating disease characterized by synovial inflammation and joint erosion. In Mexico, RA has an estimated prevalence rate of 1.6%1 and its prevalence among the working population represents a significant economic burden.2 As tuberculosis is endemic to Mexico, the global practice of screening for tuberculosis prior to initiating biologic disease-modifying antirheumatic drugs (bDMARDs) is particularly important in Mexico.3 In Mexico, RA is most commonly treated with conventional synthetic DMARDs (csDMARDs) and non-steroidal anti-inflammatory drugs.4 Only 6% of patients are treated with bDMARDs.4 Mexican College of Rheumatology (CMR) guidelines3 recommend csDMARD therapy immediately following RA diagnosis, with methotrexate as first choice, unless contraindicated. For patients with an inadequate response to csDMARDs, treatment with tumor necrosis factor inhibitors (TNFi) is suggested; tofacitinib is recommended following failed biologic treatment.3
Globally 30–40% of patients do not respond to csDMARDs, and 20–30% of patients treated with bDMARDs still have active disease,5 highlighting the need for alternative therapies to address issues of tolerability and inadequate response.
Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. The efficacy and safety of tofacitinib as monotherapy or in combination with csDMARDs have been reported in global Phase 36–11 and long-term extension (LTE)12 studies and in the overall Latin American (LA) subpopulation.13
Here we assessed tofacitinib efficacy and safety in Mexican patients with RA from global Phase 3 and LTE studies.
MethodsPatientsThis analysis included data from Mexican patients who participated in four Phase 3 studies (ORAL Scan, A392104410; ORAL Solo, A39210457; Oral Sync, A39210468; and ORAL Standard, A392106411), and one LTE study (ORAL Sequel, A392102412) of tofacitinib for the treatment of RA. Mexican patients who participated in the Phase 2 and 3 index studies and who also rolled-over into the LTE study were included in the LTE population. Data from the overall study populations were reported previously.7,8,10–12
Detailed patient inclusion criteria were reported previously.7,8,10–12 Patients were ≥18-years-old with active RA based on the American College of Rheumatology (ACR) 1987 criteria.14 Key exclusion criteria included: serious chronic or recurrent infections; evidence of active or inadequately treated latent Mycobacterium tuberculosis; history of recurrent herpes zoster (HZ), disseminated HZ or herpes simplex; hepatitis B or C; human immunodeficiency virus or other opportunistic infections; history of lymphoproliferative disorder; malignancy except adequately-treated or excised non-metastatic basal or squamous cell skin cancer or cervical carcinoma in situ.
Study design and treatmentsThe Phase 3 studies were double-blind, randomized controlled studies lasting 6–24 months. Patients had a previous inadequate response to methotrexate (ORAL Scan and ORAL Standard) or ≥1 bDMARD or csDMARD (ORAL Sync and ORAL Solo). Patients received tofacitinib 5 or 10mg twice daily (BID), or placebo, as monotherapy (ORAL Solo), or in combination with background methotrexate (ORAL Standard and ORAL Scan), or csDMARDs (ORAL Sync). ORAL Standard included an active-control arm of adalimumab 40mg administered subcutaneously once every two weeks. Data from adalimumab-treated patients are not presented due to limited sample size. In ORAL Sync, ORAL Standard and ORAL Scan, patients receiving placebo not responding at Month 3 (≥20% reduction from baseline in swollen and tender joint counts) were advanced blindly to tofacitinib 5 or 10mg BID; at Month 6, all remaining placebo patients were advanced to tofacitinib. In ORAL Solo, all patients receiving placebo advanced to tofacitinib 5 or 10mg BID at Month 3. Efficacy data from patients advanced to tofacitinib are not presented.
ORAL Sequel is an ongoing (study database was not locked as of April 2014 data cut-off; some values may change for the final locked database), open-label LTE study that enrolled patients who participated in qualifying Phase 2, or Phase 3 tofacitinib index studies. Patients initiated the LTE study with tofacitinib 5 or 10mg BID. Tofacitinib and concomitant RA medication dose adjustments were permitted for reasons of safety or inadequate response at the investigator's discretion. Baseline values for the LTE study were those of the index studies for patients enrolling in the LTE within 14 days following the index study; for all others, baseline was the start of the LTE. For this analysis, patients were analyzed in tofacitinib 5 and 10mg BID dose groups based on average total daily dose (TDD; sum of doses received divided by number of days a dose was received) in the LTE study. The 5 and 10mg BID dose groups were defined as TDD <15mg/day and TDD ≥15mg/day, respectively.
All studies were conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines, and were approved by the Institutional Review Boards and/or Independent Ethics Committees at each of the investigational centers participating in the study. All patients provided written informed consent.
Efficacy and safety endpointsEfficacy was assessed by ACR20, ACR50, and ACR70 response rates and mean changes from baseline in Disease Activity Score (erythrocyte sedimentation rate) (DAS28-4[ESR]) and Health Assessment Questionnaire-Disability Index (HAQ-DI) score.
Safety endpoints included type, severity, and frequency of adverse events (AE) and changes in clinical laboratory parameters. Incidence rates (IR; patients with events/100 patient-years) were determined for serious AEs (SAE); discontinuations due to AEs; serious infection events (SIEs); opportunistic infections (excluding tuberculosis); tuberculosis; HZ (all and serious); malignancies (excluding non-melanoma skin cancer [NMSC]); lymphoma/lymphoproliferative disorders; major adverse cardiac events (MACE); and all-cause mortality (within 30 days of last dose of study drug). Malignancy events were adjudicated by ≥2 independent, board-certified pathologists. MACE was adjudicated by a blinded, independent external committee for all Phase 3 studies, and for all events after February 2009 in the LTE study.
Statistical analysesEfficacy and safety data reported were observed case with no imputation for missing values, and were for the full analysis set (all patients randomized who received ≥1 dose of study treatment). Efficacy data up to Month 12 were pooled for Phase 3 studies (efficacy data up to Month 12 of the 2-year ORAL Scan study were included here). Efficacy is reported up to Month 36 for the LTE study due to limited sample size post-Month 36. All available safety data are presented (Phase 3: up to Month 24 [except for laboratory data where data post-Month 12 were not included due to limited sample size]; LTE: up to Month 72). Descriptive and summary statistics are presented; no formal testing was conducted for differences between treatment groups.
IRs were calculated based on the number of unique patients with events, with exposure censored at the time of the event for patients with the AE, or at time of discontinuation or data cut-off for all other patients. 95% confidence intervals (CI) were calculated using Exact Poisson adjusted for exposure.
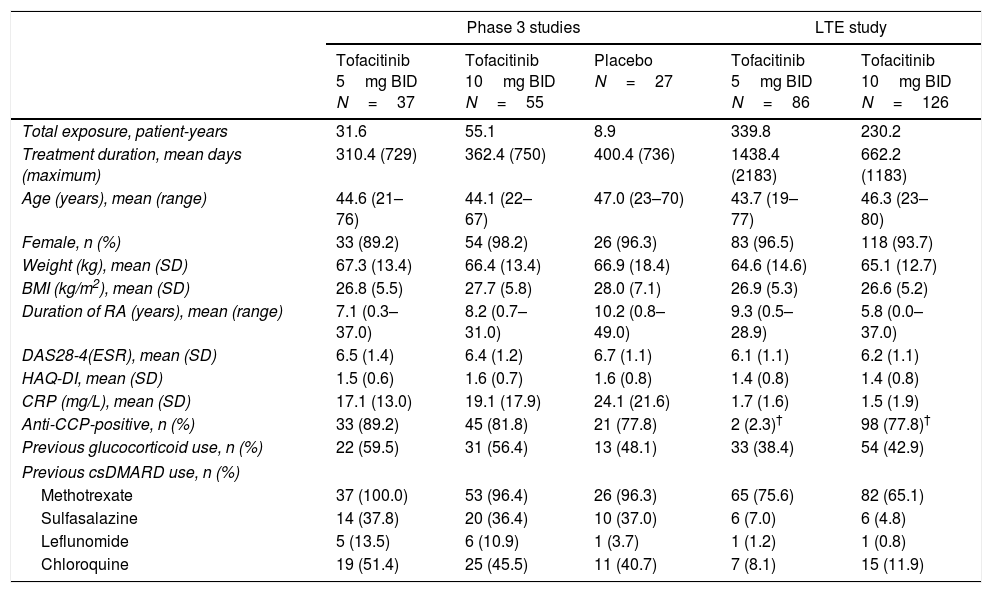
ResultsPatients119 Mexican patients were randomized across the Phase 3 studies (tofacitinib 5mg BID, N=37; tofacitinib 10mg BID, N=55; placebo advanced to tofacitinib 5mg BID, N=14; placebo advanced to tofacitinib 10mg BID, N=13). 33 (89.2%), 53 (96.4%), 13 (92.9%), and 11 (84.6%) patients completed the Phase 3 studies, respectively. In the LTE study, 212 Mexican patients were enrolled (tofacitinib 5mg BID, N=86; tofacitinib 10mg BID, N=126): 111 (52.4%) from qualifying Phase 3 index studies and 101 (47.6%) from qualifying Phase 2 index studies. Baseline demographics were generally similar across treatment groups (Table 1); mean age ranged from 43.7 to 47.0 years; the majority of patients (89.2–98.2%) were female, and mean disease duration at baseline in Phase 3 studies ranged from 7.1 to 10.2 years.
Baseline demographics and patient characteristics of the Mexican Phase 3 and LTE subpopulations by treatment group.
| Phase 3 studies | LTE study | ||||
|---|---|---|---|---|---|
| Tofacitinib 5mg BID N=37 | Tofacitinib 10mg BID N=55 | Placebo N=27 | Tofacitinib 5mg BID N=86 | Tofacitinib 10mg BID N=126 | |
| Total exposure, patient-years | 31.6 | 55.1 | 8.9 | 339.8 | 230.2 |
| Treatment duration, mean days (maximum) | 310.4 (729) | 362.4 (750) | 400.4 (736) | 1438.4 (2183) | 662.2 (1183) |
| Age (years), mean (range) | 44.6 (21–76) | 44.1 (22–67) | 47.0 (23–70) | 43.7 (19–77) | 46.3 (23–80) |
| Female, n (%) | 33 (89.2) | 54 (98.2) | 26 (96.3) | 83 (96.5) | 118 (93.7) |
| Weight (kg), mean (SD) | 67.3 (13.4) | 66.4 (13.4) | 66.9 (18.4) | 64.6 (14.6) | 65.1 (12.7) |
| BMI (kg/m2), mean (SD) | 26.8 (5.5) | 27.7 (5.8) | 28.0 (7.1) | 26.9 (5.3) | 26.6 (5.2) |
| Duration of RA (years), mean (range) | 7.1 (0.3–37.0) | 8.2 (0.7–31.0) | 10.2 (0.8–49.0) | 9.3 (0.5–28.9) | 5.8 (0.0–37.0) |
| DAS28-4(ESR), mean (SD) | 6.5 (1.4) | 6.4 (1.2) | 6.7 (1.1) | 6.1 (1.1) | 6.2 (1.1) |
| HAQ-DI, mean (SD) | 1.5 (0.6) | 1.6 (0.7) | 1.6 (0.8) | 1.4 (0.8) | 1.4 (0.8) |
| CRP (mg/L), mean (SD) | 17.1 (13.0) | 19.1 (17.9) | 24.1 (21.6) | 1.7 (1.6) | 1.5 (1.9) |
| Anti-CCP-positive, n (%) | 33 (89.2) | 45 (81.8) | 21 (77.8) | 2 (2.3)† | 98 (77.8)† |
| Previous glucocorticoid use, n (%) | 22 (59.5) | 31 (56.4) | 13 (48.1) | 33 (38.4) | 54 (42.9) |
| Previous csDMARD use, n (%) | |||||
| Methotrexate | 37 (100.0) | 53 (96.4) | 26 (96.3) | 65 (75.6) | 82 (65.1) |
| Sulfasalazine | 14 (37.8) | 20 (36.4) | 10 (37.0) | 6 (7.0) | 6 (4.8) |
| Leflunomide | 5 (13.5) | 6 (10.9) | 1 (3.7) | 1 (1.2) | 1 (0.8) |
| Chloroquine | 19 (51.4) | 25 (45.5) | 11 (40.7) | 7 (8.1) | 15 (11.9) |
Anti-CCP category data at baseline in the LTE study were missing or not collected for 82 (95.4%) and two (1.6%) patients in the 5mg BID and 10mg BID dose groups, respectively.
BID, twice daily; BMI, body mass index; CCP, cyclic citrullinated peptide; CRP, C-reactive protein; csDMARD, conventional synthetic disease-modifying antirheumatic drug; DAS28-4(ESR), Disease Activity Score (erythrocyte sedimentation rate); HAQ-DI, Health Assessment Questionnaire-Disability Index; LTE, long-term extension; RA, rheumatoid arthritis; SD, standard deviation.
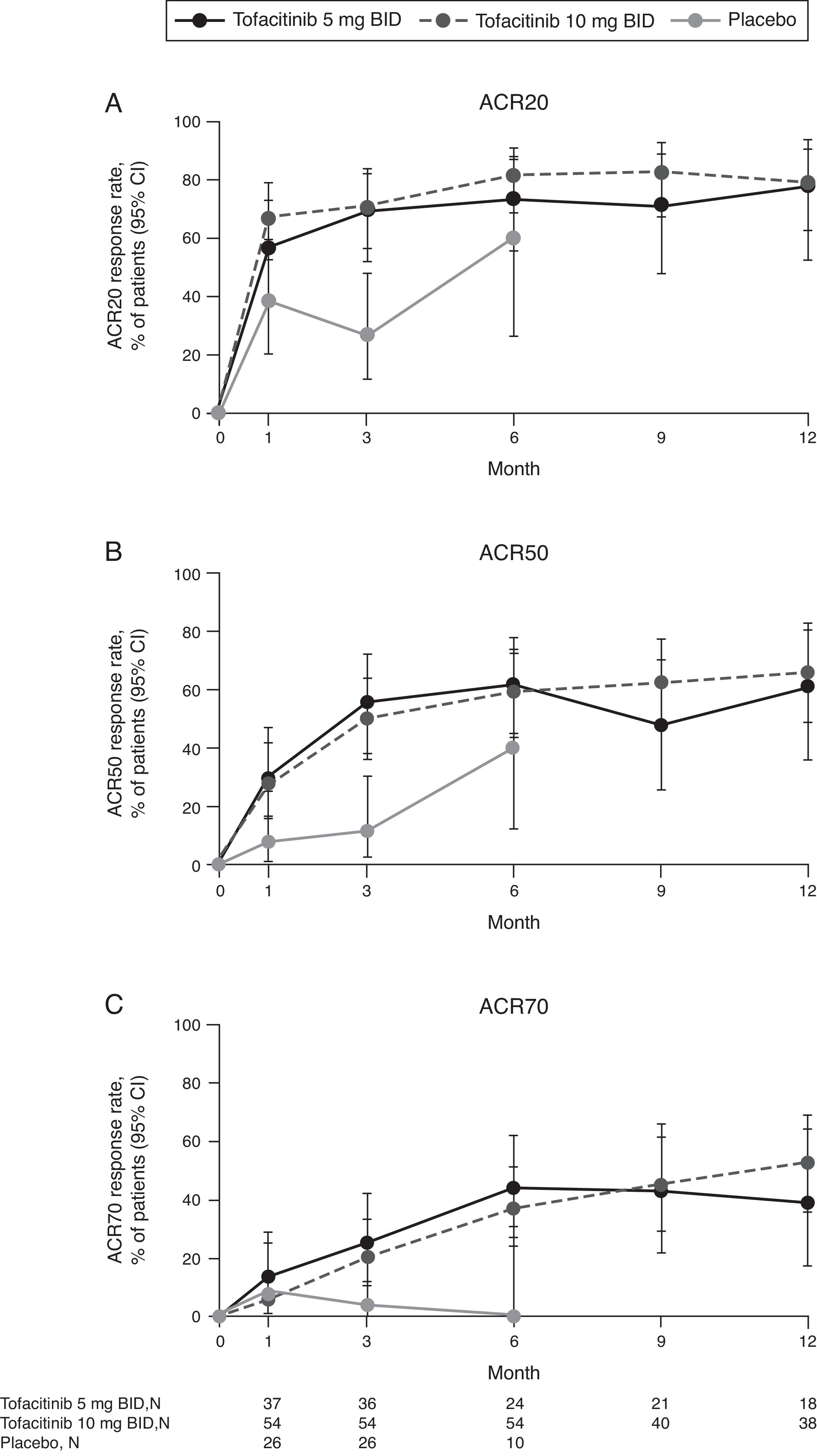
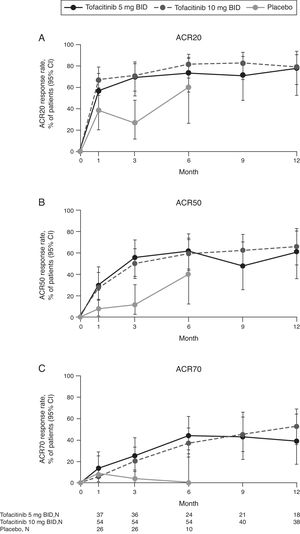
Numerically more patients who received tofacitinib 5 or 10mg BID achieved ACR20, ACR50, and ACR70 versus placebo-treated patients at Month 3 and Month 6 (Fig. 1). For ACR20 and ACR50, improvements versus placebo-treated patients were numerically greater at Month 1. The proportion of patients achieving ACR responses was generally sustained over 12 months.
(A) ACR20, (B) ACR50, and (C) ACR70 response rates (95% CI) by treatment sequence in the Mexican Phase 3 study population over time (FAS, no imputation). Data in figure are replicated in tabular form in Supplementary Table 1. Patients remaining in the placebo group at Month 6 were those with at least 20% improvement in both tender/painful and swollen joint counts at Month 3 in ORAL Scan, ORAL Sync and ORAL Standard; non-responders in the placebo group of these three studies and all placebo patients in ORAL Solo were advanced to tofacitinib treatment at Month 3 in a blinded fashion. The analysis was conducted on observed data with no imputation. The fact ‘responders’ remained in the placebo group at Month 6 with observed data being used in the analysis may contribute to the relatively high response rates for ACR20 and ACR50 in the placebo group at Month 6. ACR, American College of Rheumatology; BID, twice daily; CI, confidence interval; FAS, full analysis set; N, number of evaluable patients at time point of interest.
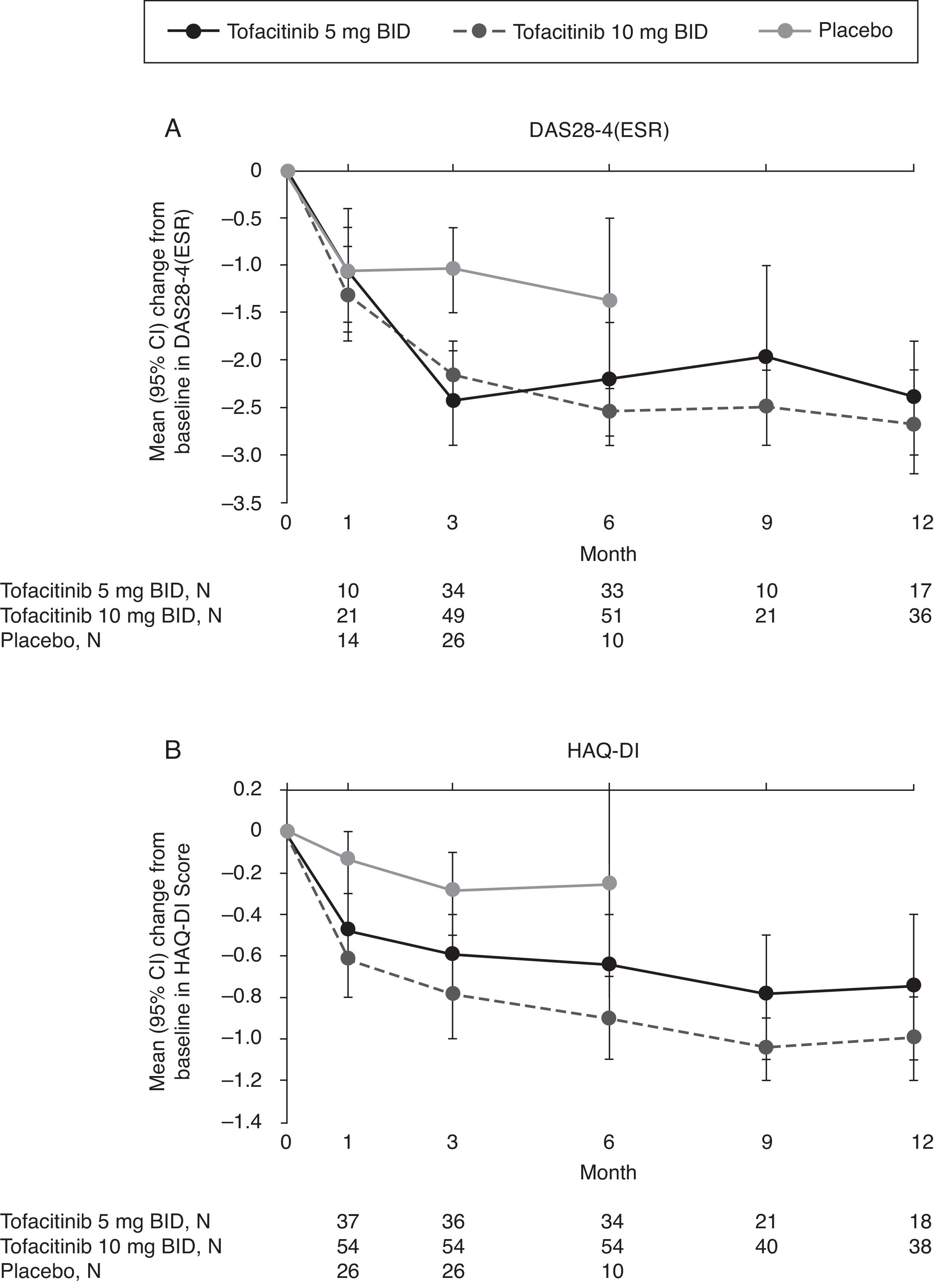
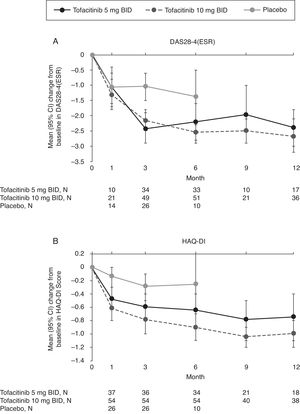
Mean changes from baseline in DAS28-4(ESR) were numerically greater with tofacitinib versus placebo at Month 3 and Month 6 (Fig. 2A). Improvements in DAS28-4(ESR) were sustained with tofacitinib treatment up to Month 12.
(A) Mean (95% CI) change from baseline in DAS28-4(ESR) and (B) mean (95% CI) change from baseline in HAQ-DI score by treatment sequence in the Mexican Phase 3 study population over time (FAS, no imputation). Data in figure are replicated in tabular form in Supplementary Table 2. Patients remaining in the placebo group at Month 6 were those with at least 20% improvement in both tender/painful and swollen joint counts at Month 3 in ORAL Scan, ORAL Sync, and ORAL Standard; non-responders in the placebo group of these three studies and all placebo patients in ORAL Solo were advanced to tofacitinib treatment at Month 3 in a blinded fashion. The analysis was conducted on observed data with no imputation. BID, twice daily; CI, confidence interval; DAS28-4(ESR), Disease Activity Score (erythrocyte sedimentation rate); FAS, full analysis set; HAQ-DI, Health Assessment Questionnaire-Disability Index; N, number of evaluable patients at time point of interest.
Numerically greater mean improvements from baseline HAQ-DI were seen with both doses of tofacitinib versus placebo at Months 1, 3, and 6 (Fig. 2B). Improvements in HAQ-DI were sustained up to Month 12 with tofacitinib.
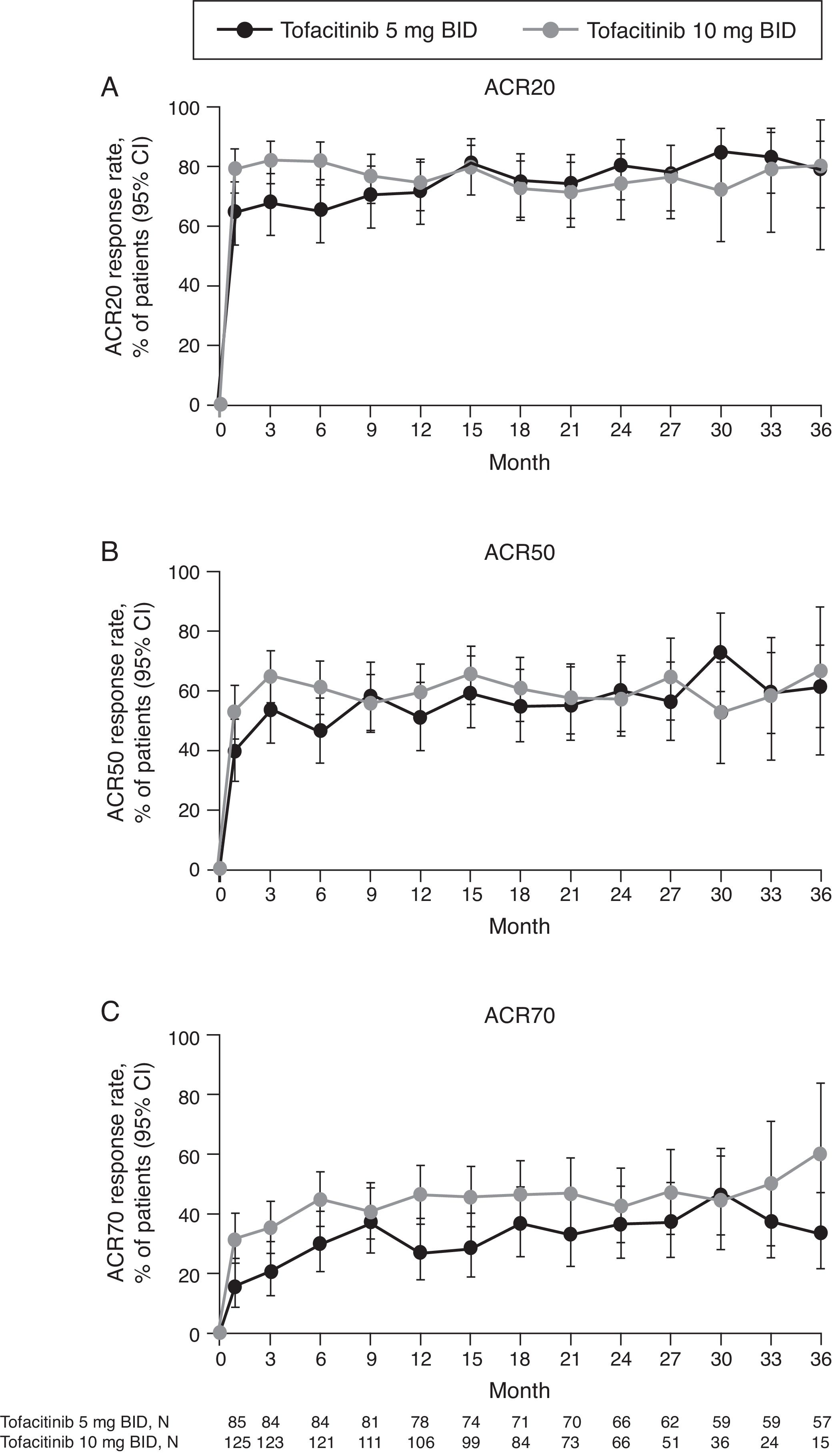
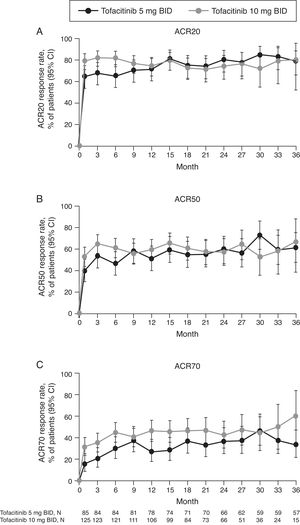
LTE studyACR response rates at Month 1 of the LTE study were maintained up to Month 36 (Fig. 3) with both tofacitinib doses. ACR response rates were initially numerically greater for tofacitinib 10mg BID versus tofacitinib 5mg BID. After Month 9 in the LTE, ACR20, and ACR50 response rates were generally similar between tofacitinib 5 and 10mg BID groups. ACR70 response rates were generally higher with tofacitinib 10mg BID versus tofacitinib 5mg BID up to Month 36.
(A) ACR20, (B) ACR50, and (C) ACR70 response rates (95% CI) in the Mexican LTE study population over time (FAS, no imputation). Data in figure are replicated in tabular form in Supplementary Table 3. ACR, American College of Rheumatology; BID, twice daily; CI, confidence interval; FAS, full analysis set; LTE, long-term extension; N, number of evaluable patients at time point of interest.
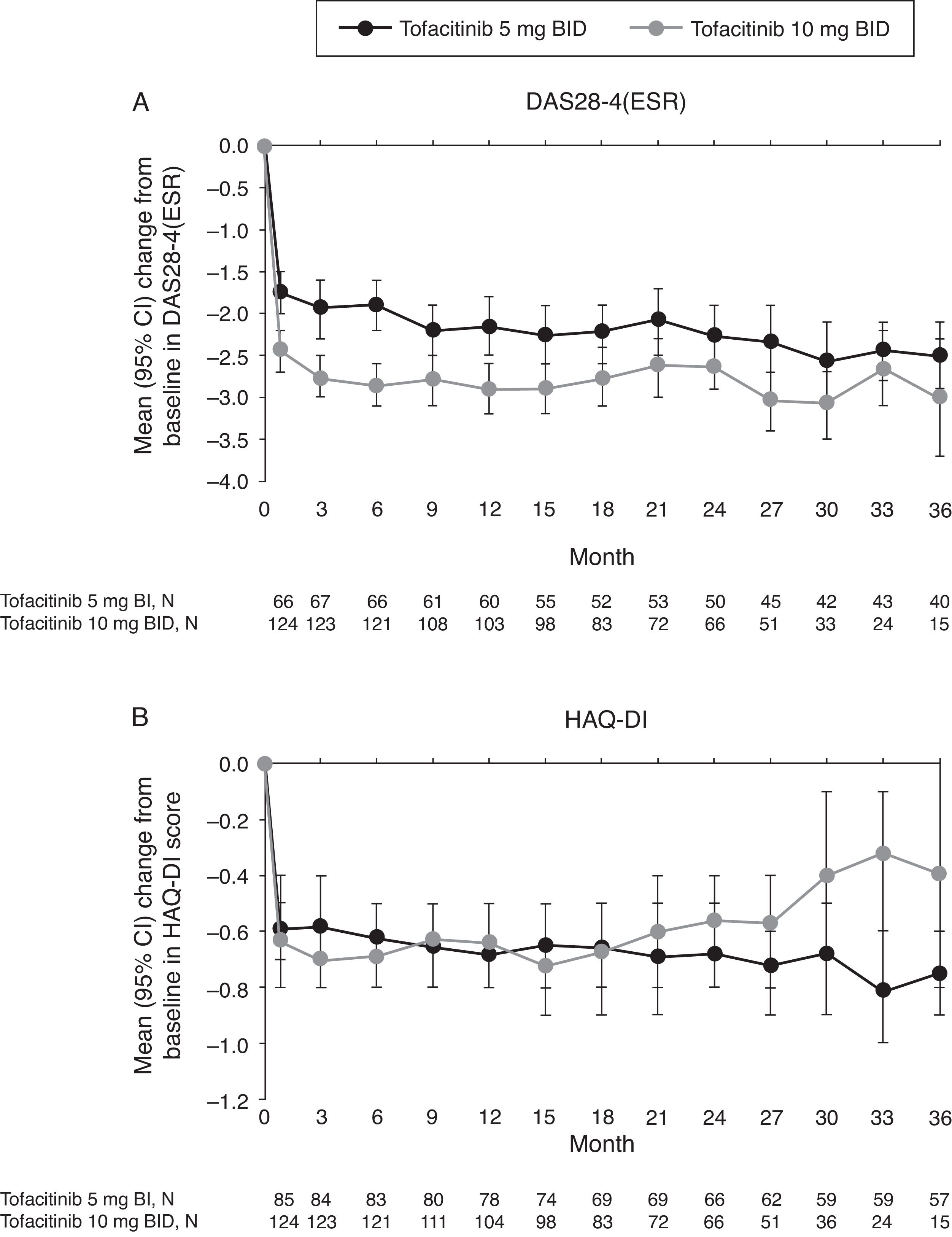
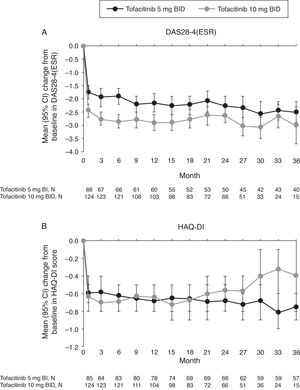
Mean improvements from baseline in DAS28-4(ESR) observed at Month 1 of the LTE study were sustained with both tofacitinib doses up to Month 36 (Fig. 4A). Improvement in DAS28-4(ESR) was numerically greater with tofacitinib 10mg BID versus tofacitinib 5mg BID at all time points.
(A) Mean (95% CI) change from baseline in DAS28-4(ESR) and B) mean (95% CI) change from baseline in HAQ-DI score in the Mexican LTE study population over time (FAS, no imputation). Data in figure are replicated in tabular form in Supplementary Table 4. BID, twice daily; CI, confidence interval; DAS28-4(ESR), Disease Activity Score (erythrocyte sedimentation rate); FAS, full analysis set; HAQ-DI, Health Assessment Questionnaire-Disability Index; LTE, long-term extension; N, number of evaluable patients at time point of interest.
Patients treated with tofacitinib 5 and 10mg BID had similar improvements from baseline in HAQ-DI at Month 1 of the LTE study that were generally sustained to Month 36 (Fig. 4B).
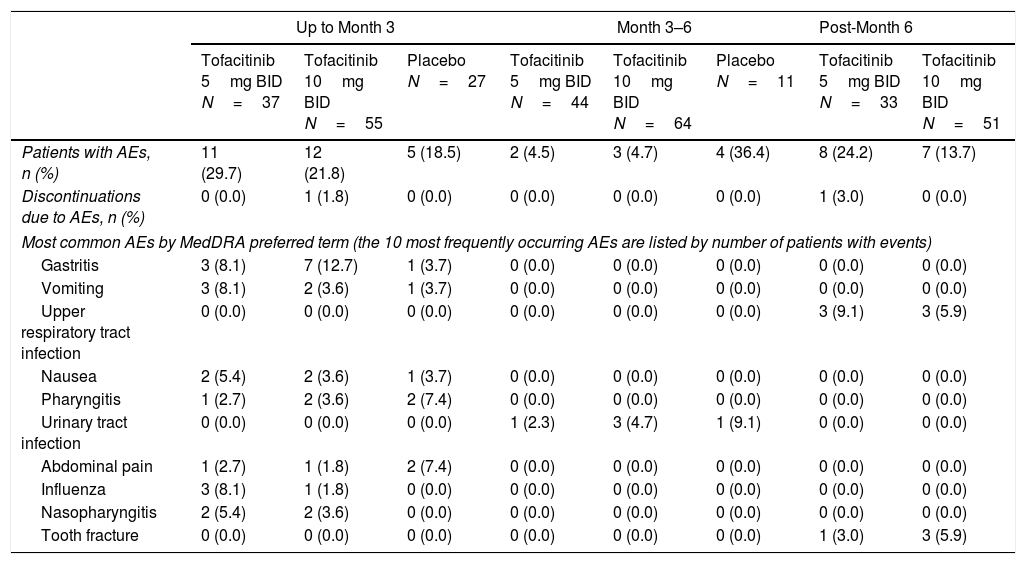
SafetyPhase 3 studiesUp to Month 3, 29.7% of patients had AEs with tofacitinib 5mg BID, 21.8% with tofacitinib 10mg BID, and 18.5% with placebo (Table 2); infections occurred in 16.2% and 9.1% of patients receiving tofacitinib 5mg BID and tofacitinib 10mg BID, respectively, versus 7.4% with placebo. During Months 3–6, 4.5% and 4.7% of patients treated with tofacitinib 5 and 10mg BID, respectively, had AEs. Post-Month 6, 24.2% and 13.7% of patients treated with tofacitinib 5 and 10mg BID had AEs, respectively. The most frequently occurring AEs by treatment period are listed in Table 2. There was one pregnancy during the Phase 3 studies (tofacitinib 10mg BID group).
Summary of AEs, discontinuations due to AEs, and most common AEs in the Mexican Phase 3 study subpopulation by treatment period.
| Up to Month 3 | Month 3–6 | Post-Month 6 | ||||||
|---|---|---|---|---|---|---|---|---|
| Tofacitinib 5mg BID N=37 | Tofacitinib 10mg BID N=55 | Placebo N=27 | Tofacitinib 5mg BID N=44 | Tofacitinib 10mg BID N=64 | Placebo N=11 | Tofacitinib 5mg BID N=33 | Tofacitinib 10mg BID N=51 | |
| Patients with AEs, n (%) | 11 (29.7) | 12 (21.8) | 5 (18.5) | 2 (4.5) | 3 (4.7) | 4 (36.4) | 8 (24.2) | 7 (13.7) |
| Discontinuations due to AEs, n (%) | 0 (0.0) | 1 (1.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.0) | 0 (0.0) |
| Most common AEs by MedDRA preferred term (the 10 most frequently occurring AEs are listed by number of patients with events) | ||||||||
| Gastritis | 3 (8.1) | 7 (12.7) | 1 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vomiting | 3 (8.1) | 2 (3.6) | 1 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Upper respiratory tract infection | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (9.1) | 3 (5.9) |
| Nausea | 2 (5.4) | 2 (3.6) | 1 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pharyngitis | 1 (2.7) | 2 (3.6) | 2 (7.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Urinary tract infection | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) | 3 (4.7) | 1 (9.1) | 0 (0.0) | 0 (0.0) |
| Abdominal pain | 1 (2.7) | 1 (1.8) | 2 (7.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Influenza | 3 (8.1) | 1 (1.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nasopharyngitis | 2 (5.4) | 2 (3.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Tooth fracture | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.0) | 3 (5.9) |
AE, adverse event; BID, twice daily; MedDRA, Medical Dictionary for Regulatory Activities.
SAEs occurred in two patients (5.4%) receiving tofacitinib 5mg BID (one event was treatment-related [investigator-determined]); three patients (5.5%) receiving tofacitinib 10mg BID (five separate events, two treatment-related); and two patients (14.3%) from the placebo to tofacitinib 5mg BID treatment sequence (three separate events, two treatment-unrelated events during placebo treatment phase, one treatment-related during active treatment). Discontinuation due to AEs was infrequent for all treatment groups during all three time periods in Phase 3 studies (Table 2). There were no SIEs, tuberculosis, malignancies or adjudicated cardiovascular events. There were three patients with HZ; two (3.6%) with tofacitinib 10mg BID and one (3.7%) with placebo. No Mexican patients died during Phase 3 studies.
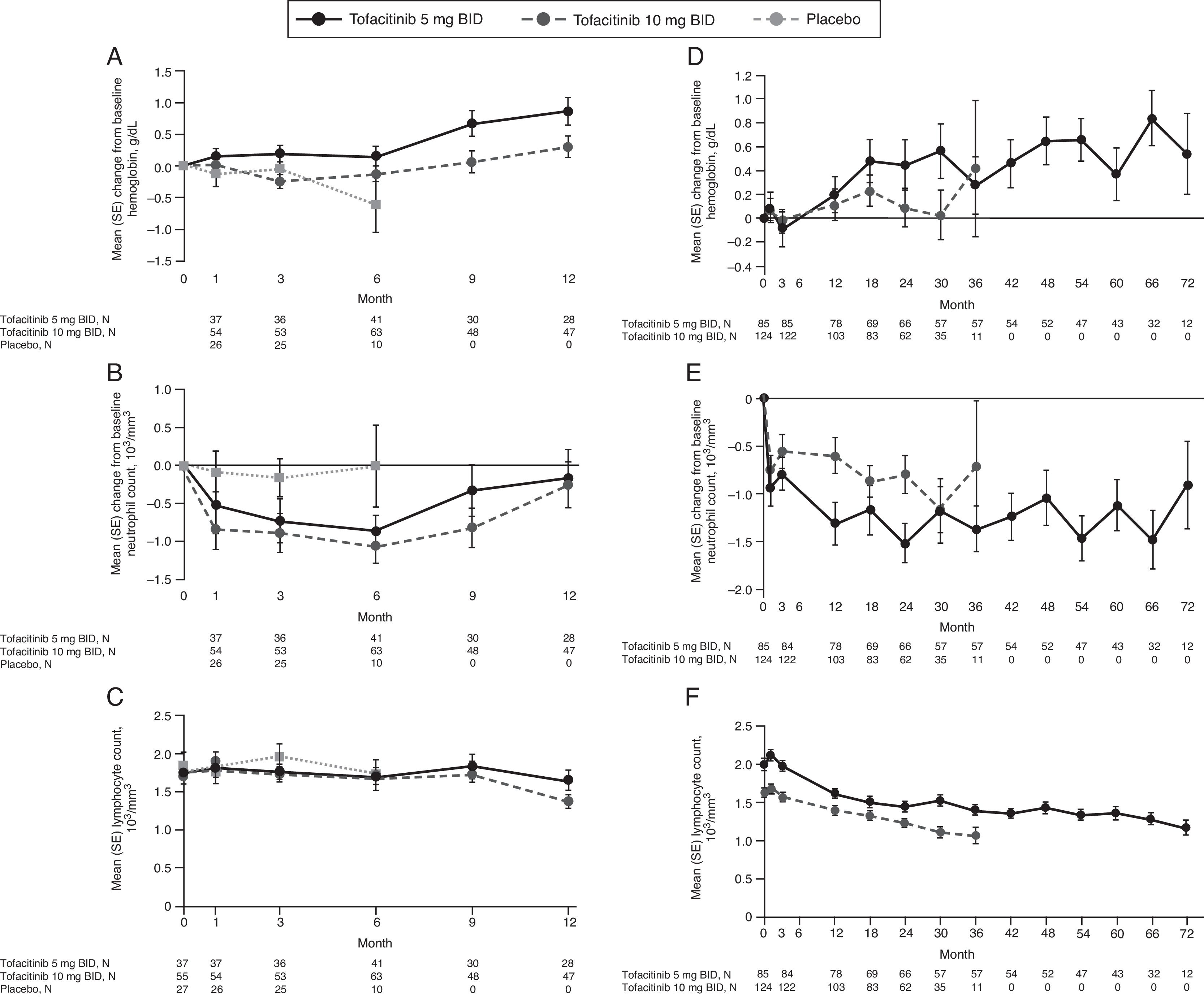
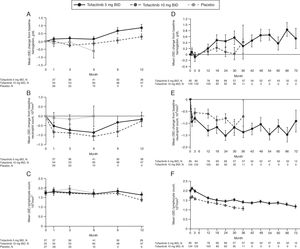
There was a slight increase from baseline in hemoglobin with tofacitinib treatment up to Month 12, and slight decreases from baseline in neutrophil and lymphocyte counts (Fig. 5A–C). 31, 41, 12, and nine patients in the tofacitinib 5mg BID, tofacitinib 10mg BID, placebo to tofacitinib 5mg BID, and placebo to tofacitinib 10mg BID sequences, respectively, had confirmed lymphopenia (two sequential absolute lymphocyte counts <2000cells/mm3) during the 12-month Phase 3 study period; none had SIEs within 30 days of the lowest lymphocyte cell count.
Mean laboratory parameters over time in the Mexican Phase 3 (A–C) and LTE (D–F) study populations. (A) and (D) change from baseline in hemoglobin; (B) and (E) change from baseline absolute neutrophil count; C) and F) absolute lymphocyte count. Data in figure are replicated in tabular form in Supplementary Table 5A (panels A–C) and Supplementary Table 5B (panels D–F). Data for Phase 3 is only presented up to Month 12 due to low sample size post-Month 12 BID, twice daily; LTE, long-term extension; N, number of evaluable patients at time point of interest; SE, standard error.
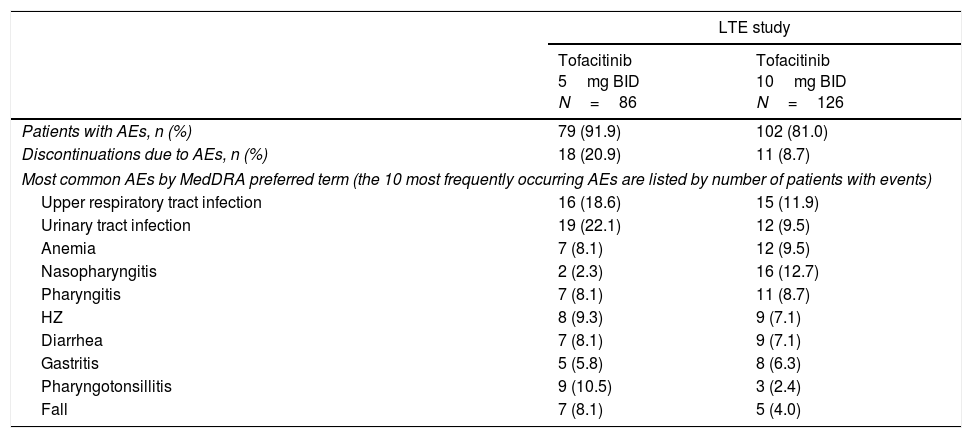
Up to Month 72 of the LTE study, AEs occurred in 91.9% and 81.0% of patients receiving tofacitinib 5 and 10mg BID, respectively (Table 3).
Summary of AEs, discontinuations due to AEs, and most common AEs up to Month 72 in the Mexican LTE study subpopulation.
| LTE study | ||
|---|---|---|
| Tofacitinib 5mg BID N=86 | Tofacitinib 10mg BID N=126 | |
| Patients with AEs, n (%) | 79 (91.9) | 102 (81.0) |
| Discontinuations due to AEs, n (%) | 18 (20.9) | 11 (8.7) |
| Most common AEs by MedDRA preferred term (the 10 most frequently occurring AEs are listed by number of patients with events) | ||
| Upper respiratory tract infection | 16 (18.6) | 15 (11.9) |
| Urinary tract infection | 19 (22.1) | 12 (9.5) |
| Anemia | 7 (8.1) | 12 (9.5) |
| Nasopharyngitis | 2 (2.3) | 16 (12.7) |
| Pharyngitis | 7 (8.1) | 11 (8.7) |
| HZ | 8 (9.3) | 9 (7.1) |
| Diarrhea | 7 (8.1) | 9 (7.1) |
| Gastritis | 5 (5.8) | 8 (6.3) |
| Pharyngotonsillitis | 9 (10.5) | 3 (2.4) |
| Fall | 7 (8.1) | 5 (4.0) |
AE, adverse event; BID, twice daily; HZ, herpes zoster; LTE, long-term extension; MedDRA, Medical Dictionary for Regulatory Activities.
The most frequently occurring AEs in either dose group were urinary tract infection, upper respiratory tract infection, and nasopharyngitis (Table 3). Infections occurred in 74.4% and 57.9% of patients receiving tofacitinib 5 and 10mg BID, respectively. There was one pregnancy in the tofacitinib 5mg BID group.
Three Mexican patients died during the LTE study (without 30-day rule): two patients receiving tofacitinib 5mg BID; and one receiving tofacitinib 10mg BID. Causes of death were: hepatic failure and sepsis; and respiratory failure and chronic obstructive pulmonary disease for patients treated with tofacitinib 5mg BID; and synovial sarcoma and metastases to lung for the patient receiving tofacitinib 10mg BID.
In the LTE study, mean increases from baseline in hemoglobin levels and mean decreases from baseline in neutrophil count were observed with both doses of tofacitinib, and were stable with longer treatment duration (Fig. 5D and E). Mean lymphocyte count increased up to Month 1, returned to baseline levels by Month 3, and gradually decreased thereafter (Fig. 5F). Of the 80 and 115 patients with confirmed lymphopenia in the tofacitinib 5 and 10mg BID groups, respectively, none had SIEs within 30 days of the lowest lymphocyte cell count.
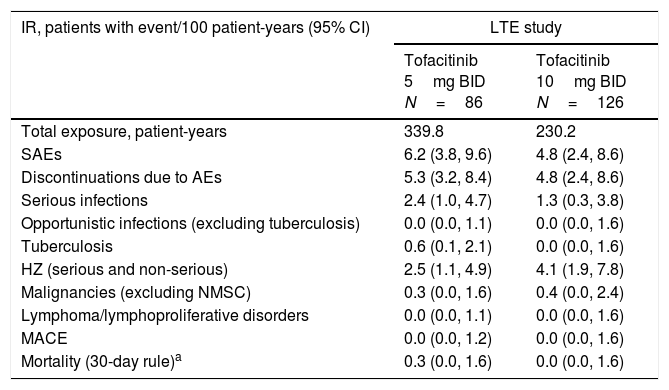
IRs and 95% CIs for SAEs, discontinuations due to AEs, and other events of special interest are presented in Table 4. IRs for all safety events of special interest were generally similar between tofacitinib 5 and 10mg BID, with overlapping CIs (Table 4). However, tofacitinib exposure for the two groups was low. There were no opportunistic infections (excluding tuberculosis) or MACE in either treatment group. There were two cases of tuberculosis in the tofacitinib 5mg BID group.
IRs (patients with event/100 patient-years) for AEs of special interest by treatment group in the Mexican LTE study subpopulation.
| IR, patients with event/100 patient-years (95% CI) | LTE study | |
|---|---|---|
| Tofacitinib 5mg BID N=86 | Tofacitinib 10mg BID N=126 | |
| Total exposure, patient-years | 339.8 | 230.2 |
| SAEs | 6.2 (3.8, 9.6) | 4.8 (2.4, 8.6) |
| Discontinuations due to AEs | 5.3 (3.2, 8.4) | 4.8 (2.4, 8.6) |
| Serious infections | 2.4 (1.0, 4.7) | 1.3 (0.3, 3.8) |
| Opportunistic infections (excluding tuberculosis) | 0.0 (0.0, 1.1) | 0.0 (0.0, 1.6) |
| Tuberculosis | 0.6 (0.1, 2.1) | 0.0 (0.0, 1.6) |
| HZ (serious and non-serious) | 2.5 (1.1, 4.9) | 4.1 (1.9, 7.8) |
| Malignancies (excluding NMSC) | 0.3 (0.0, 1.6) | 0.4 (0.0, 2.4) |
| Lymphoma/lymphoproliferative disorders | 0.0 (0.0, 1.1) | 0.0 (0.0, 1.6) |
| MACE | 0.0 (0.0, 1.2) | 0.0 (0.0, 1.6) |
| Mortality (30-day rule)a | 0.3 (0.0, 1.6) | 0.0 (0.0, 1.6) |
In this analysis of Mexican patients from the tofacitinib global RA program, tofacitinib reduced the signs and symptoms of RA and improved physical function, when administered as monotherapy or in combination with csDMARDs up to 12 Months in Phase 3, and up to 36 Months in the LTE study. Although formal statistical comparisons were not conducted, tofacitinib efficacy in Mexican patients was generally consistent with that observed in the global7,8,10–12 and LA13 Phase 3 and LTE analyses; however, a greater proportion of Mexican patients were female and Mexican patients were typically younger. The small sample sizes in the subpopulations precluded statistical comparison between treatment groups and formal conclusions being drawn regarding the efficacy and safety of tofacitinib. These factors should be considered in interpreting outcomes.
The safety profile of tofacitinib in Mexican patients was generally similar with respect to the Phase 3 and LTE global7,8,10–12 and LA analyses,13 including low and comparable IRs of opportunistic infections (excluding tuberculosis), tuberculosis, malignancies (excluding NMSC), and MACE in LTE study analyses. As in the global LTE study,12 there were increases in hemoglobin levels and decreases in absolute neutrophil and lymphocyte counts in the Mexican LTE study population.
Patients with RA have a greater risk of infections, including tuberculosis, that increases with use of immunosuppressive agents and bDMARDs.15 In LA, registry data on the use of bDMARDs16,17 also indicated increased risk of infections and tuberculosis with therapy. In this analysis, no SIEs or tuberculosis occurred during Phase 3 studies and there were no opportunistic infections and only two cases of tuberculosis reported during the LTE study. However, it should be acknowledged that the Mexican subpopulation is small, and patients participating in tofacitinib Phase 3 and LTE studies were screened for active or latent tuberculosis.
In the global RA program, IRs for HZ were generally higher with tofacitinib versus placebo and bDMARDs18; the overall HZ risk was particularly increased in Japanese and Korean patients. The IRs (unique patients with events/100 patient-years) of HZ in Mexican tofacitinib-treated patients in the LTE analysis presented here were numerically lower with tofacitinib 5mg BID and similar with 10mg BID compared with the global LTE analysis (4.2 [95% CI 3.5, 5.0] and 4.5 [3.8, 5.4] for tofacitinib 5 and 10mg BID, respectively),12 but higher than those reported in analyses of registry data for bDMARD-treated patients with RA in the UK19 (1.6 events/100 patient-years), US20 (1.2 events/100 patient-years) and Germany21 (1.1 events/100 patient-years). In interpreting the rate of HZ observed in these analyses, it should be noted that patients with history of recurrent HZ, disseminated HZ or herpes simplex were not permitted to be enrolled in these studies.
In light of CMR recommendations,3 tofacitinib's oral administration route may be of benefit in reducing the number of clinic visits required by patients. Moreover, unlike bDMARDs, which are administered via injection and with which patients may lose response owing to acquired drug resistance,22 tofacitinib is an oral small molecule therapy and patients with RA may prefer treatment in the form of a tablet over injectable therapies.23 Accordingly, tofacitinib offers an alternative for Mexican patients with RA who failed treatment with csDMARDs. A further potential benefit of a small molecule therapy for RA in the Mexican healthcare setting may be a reduction in administrative costs owing to fewer required patient visits to the clinic and independence from temperature-controlled supply chains. However, an evaluation of the pharmacoeconomic impact of tofacitinib for RA therapy was not included in this study, and additional studies should be performed to assess this.
Compared to the tofacitinib global study population, the number of patients, tofacitinib exposure and treatment duration were lower in these subpopulation analyses which may explain the lower rates of long-latency AEs such as MACE and malignancies. Interpretation of the Phase 3 data in this analysis was limited by pooling of data from studies with different patient populations, study designs, and methodologies. Fewer patients were enrolled in the placebo groups of Phase 3 studies and consequently patient exposure was lower. In interpreting the Phase 3 efficacy analyses, it should also be noted that patients remaining in the placebo group at Month 6 were those from ORAL Scan, Sync, and Standard who had at least 20% improvement in both tender/painful and swollen joint counts at Month 3 and this may explain the relatively high response rate observed for some efficacy endpoints (e.g. ACR20/50) in the placebo group at Month 6. Interpretation of the data from the LTE study was limited by its observational nature. Furthermore, the tolerability and efficacy of tofacitinib have already been demonstrated in patients enrolling in the LTE study through their index study participation. Dose adjustments were also permitted in the LTE, limiting comparison between tofacitinib doses. Nonetheless, due scarce long-term real-life data, LTE data and real-world data studies24 are important in evaluating tofacitinib in the Mexican subpopulation.
Tofacitinib 5mg BID and 10mg BID showed improvement in efficacy endpoints which were sustained with long-term therapy up to 36 months post-index study. The safety profile of tofacitinib in Mexican patients with RA was consistent with that of the LA and global populations.
Key messages- •
Sustained efficacy in reducing signs and symptoms of RA was observed up to 36 months in Mexican patients treated with tofacitinib in the LTE study.
- •
The safety profile of tofacitinib in the Mexican subpopulation was consistent with that observed in the global and overall Latin American Phase 3 and LTE study populations.
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestR Burgos-Vargas has received research grants, consultancy fees, and/or speakers’ fees from AbbVie, Janssen, Lilly, Novartis, Pfizer Inc., and UCB.
MH Cardiel has received research grants, consultancy fees, and speakers’ fees from AbbVie, Astellas, Astra Zeneca, Bristol-Myers Squibb, Infinity, Janssen, Lilly, Merck-Serono, Pfizer Inc, Roche, and UCB.
D Xibillé has received consultancy fees from Pfizer Inc. and payment for clinical studies from AstraZeneca, Bristol-Myers Squibb, Janssen, Pfizer Inc., and UCB.
C Pacheco-Tena has received payment for clinical studies, consultancy fees, or speaker fees from AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, Lilly, Merck-Serono, Novo Nordisk, Pfizer Inc, Roche, Sanofi, UCB, and Vertex.
V Pascual-Ramos has nothing to disclose.
C Abud-Mendoza has received consultancy fees and speaker fees from Bristol-Myers Squibb, Pfizer Inc, and Roche and speaker fees from Merck-Serono and UCB.
EY Mahgoub, H Fan, R Rojo, and K Santana are employees and shareholders of Pfizer Inc. MU Rahman and EG García were employees and shareholders of Pfizer Inc at the time the analyses were conducted.
This study was sponsored by Pfizer Inc. Medical writing support under the guidance of the authors was provided by Daniel Binks, PhD, of Complete Medical Communications and funded by Pfizer Inc.