To evaluate the efficacy and safety of belimumab in patients with Primary Sjögren's syndrome (pSS).
MethodsThe search included manuscripts assessing the efficacy or safety of belimumab in patients with pSS (American-European Consensus Criteria 2002) published between 2004 and 2017 in MEDLINE, EMBASE or Cochrane databases. Two reviewers independently selected the articles, extracted data and evaluated the quality of the evidence following Scottish Intercollegiate Guidelines Network (SIGN) recommendation grades.
ResultsOut of 135 citations, only 3 articles were included. All of them publishing results from the same study at different time points including 28 patients. At week 28 improvement was reported for visual analogue scale (VAS) dryness score and glandular manifestations in 37% and 77% of patients, respectively, which persisted at week 52 (W52). Belimumab was well tolerated and safely administered.
ConclusionPublished evidence to determine the efficacy of belimumab in pSS is limited. Belimumab seems to be effective to reduce systemic activity, parotid enlargement, lymphadenopathies, articular manifestation and B cell biomarkers.
Evaluar la eficacia y la seguridad de belimumab en pacientes con síndrome de Sjögren primario (SSp).
MétodosLa búsqueda incluyó manuscritos que evaluaban la eficacia o seguridad de belimumab en pacientes con SSp (Criterios Europeo-Americanos del 2002) publicados entre 2004 y 2007 en MEDLINE, EMBASE o Cochrane database. Dos revisores independientes seleccionaron los artículos, extrajeron los datos y evaluaron la calidad de la evidencia según los grados de recomendación de la Scottish Intercollegiate Guidelines Network (SIGN).
ResultadosDe 135 artículos se incluyeron 3. Todos publicaban resultados del mismo estudio en diferentes momentos, incluyéndose 28 pacientes. En la semana 28 presentaban una mejoría en la puntuación de sequedad en la escala analógica visual (VAS) y en las manifestaciones glandulares un 37 y 77% de los pacientes, respectivamente, que persistieron en la 52. La administración de belimumab fue segura y bien tolerada.
ConclusiónBelimumab parece ser efectivo para reducir la actividad sistémica, el aumento parotídeo, las linfadenopatías, las manifestaciones articulares y los biomarcadores de células B, aunque con evidencia limitada.
Primary Sjögren's syndrome (pSS) is a relatively common systemic autoimmune rheumatic disease, in which lymphocytic infiltration of salivary and lacrimal glands leads to immune-mediated secretory dysfunction.1 It usually affects predominantly middle-aged women, typically in the fourth to sixth decades of life.2 In addition to salivary and lacrimal dysfunction, patients with pSS may present a large number of extraglandular manifestations, either as the presenting manifestation or during the disease process.3 The clinical picture is also associated with an 11-fold higher risk of developing haematological cancers, mainly B cell lymphomas.4
Nowadays, the burden of this disease is substantial because of the lack of effective therapeutic options. The pathogenesis is multifactorial and implies a dysregulation of several immunity pathways. Patients with pSS have shown an increased expression of the cytokine B cell activating factor (BAFF), also called B-Lymphocyte Stimulator (BLys), in serum and salivary glands compared with controls. This factor stimulates B cell growth and hyperactivity, therefore suggesting an important role of these cells in the pathogenesis of this disease.5–7 Moreover, in patients with pSS serum levels of BAFF (BLys) have been found to be associated with disease activity and with lymphoproliferative complications.8 Belimumab is a human monoclonal antibody that inhibits BAFF. It has shown to be effective in systemic lupus erythematosus (SLE) and therefore it is approved for its treatment in Europe and North America.9 Based on the pathogenesis of pSS, belimumab could also be effective improving symptoms and signs of this disease. However, the efficacy of belimumab in pSS is still unclear.
The objective of this systematic review was to evaluate the efficacy and safety of belimumab in patients with pSS for the treatment of dryness, glandular and systemic manifestations.
Materials and methodsA systematic review of the literature to identify all publications including patients with pSS and treatment with belimumab was conducted as part of the development of the Recommendations for the Use of Biological Therapies in Primary Sjögren's Syndrome of the Spanish Society of Rheumatology.
The clinical question was formulated according to PICO method10 as follows: Population: patients with pSS according to American-European Consensus Criteria 2002; Intervention: belimumab; Control: synthetic or biologic disease-modifying antirheumatic drugs (DMARDs), corticosteroids, ursodesoxicolic acid or placebo; Outcome: efficacy in dryness, glandular and systemic manifestations measured through validated indexes.
During all the systematic literature review, the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) consensus was followed.11
Search strategyArticles published between 2004 and 2017 in MEDLINE, EMBASE or Cochrane database were searched. The search strategy included the following MeSH terms and synonyms: Sjogren's Syndrome, belimumab, biologic disease modifying antirheumatic drug. The searches were conducted with a language restriction (English, French and Spanish).
Selection criteriaWe included randomized controlled trials (RTCs), systematic review, meta-analysis and prospective observational studies of adult patients. Only studies with more than 3 months of follow-up and inclusion of at least 10 patients were selected.
We considered the following outcomes: dryness, evaluated through visual analogue scale (VAS), Schirmer test and unstimulated salivary flow; musculoskeletal pain and fatigue, evaluated through VAS; biologic biomarkers; quality of life, measured through the Short Form-36 (SF36) health survey; disease activity, evaluated through the European League Against Rheumatism (EULAR) Sjögren's Disease Activity Index (ESSDAI); symptom perception, evaluated through the EULAR Sjögren's Syndrome Patient Report Index (ESSPRI) and glandular affection. We also considered the adverse events reported by authors.
Two authors (H.S.P. and N.A.R.) independently screened titles and abstracts and evaluated the eligibility of the identified studies, using a third reviewer (VNC) for consensus in case of discrepancies. The selection was made using the EndNote X7s software. Once unrelated articles were excluded, the full report of all the selected studies was reviewed. Subsequently, articles non-fulfilling all selection criteria were excluded. In addition, a hand search of articles found in the reference lists of the included articles was made and abstracts and the congress abstracts online (from 2016) from the EULAR and the American College of Rheumatology (ACR) were searched.
Data extraction and analysisThe same two reviewers independently extracted data using a specific extraction data sheet designed for this purpose. A qualitative analysis of the data was performed and supported by evidence tables. The collected data included publication details, study design, characteristics of patients, outcomes and adverse events.
The quality of the study was evaluated according the Scottish Intercollegiate Guidelines Network (SIGN).12
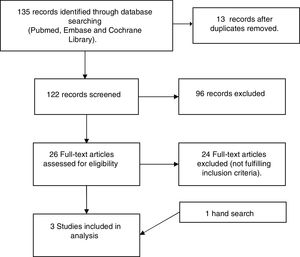
ResultsSearch resultsFig. 1 depicts the results for the search and selection process. The search captured 135 references throughout database searching. After title/abstract screening, 26 articles were retrieved for full text review and 2 were included. One hand search was also included.
The three articles included reported different results from the same study (Phase II Open-label BELISS study) at different time points13–15 (Table 1). This study included 28 patients, all of them women, with a mean (standard) age of 49.5 (16.5) years and with a mean (SD) disease duration of 5.7 (5.6) years. Out of these patients, 71.4% patients had systemic complications, 6.7% had lymphoma and 10.3% had cryoglobulinemia (Table 2).
Table of evidence of studies: design and outcomes.
| Authors | Mariette et al. | De Vita et al. | Quartuccio et al. |
|---|---|---|---|
| Design | Phase II Open-label BELISS study | Phase II Open-label BELISS study | Prospective study follow-up after the end of BELISS study |
| No. of patients evaluated | 30 | 19 (15) | 13 |
| Intervention | 10mg/kg belimumab on week 0,2,4 and every 4 weeks | 10mg/kg belimumab every 4 week | Withdrawal of treatment with belimumab |
| Follow-up period (weeks) | 28 | 52 | 48 |
| Outcome | Primary end point: Improvement in at least two of following items: ≥ 30% reduction in dryness score on a VAS, ≥30% reduction in fatigue score on a VAS, ≥30% reduction in musculoskeletal pain score on a VAS, ≥30% reduction in systemic activity score o a VAS assessed by the physician and/or ≥25% reduction in serum levels of any of the B-cell activation biomarkers or ≥25% increase in C4 level. | Change on composite index of disease activity (ESSDAI), composite index of symptom perception (ESSPRI), glandular affection and on serum biomarkers (Igs, RF and BLyS) after withrawal of belimumab therapy | |
| Level of evidence | 3 | 3 | 3 |
VAS: Visual analogue scale.
Baseline characteristics of patients included in the selected studies.
| Authors | Mariette et al. | De Vita et al. | Quartuccio et al. |
|---|---|---|---|
| % Women | 100 | 100 | 100 |
| No. of patients | 30 | 19 (15) | 13 |
| Mean age (years), mean (SD) | 49.5 (16.5) | 40.2 (11.8) | 54 (15) |
| Mean disease duration (years), mean (SD) | 5.7 (5.6) | 5.9 (5.7) | 12.7 (5.2) |
| Anti-SSA positivity (%) | 96.7 | 93.3 | 100 |
| Anti-SSB positivity (%) | 73.3 | 93.3 | 92.3 |
| Recent onsetdisease (%) | 34.5 | 26.7 | NA |
| Systemic complications (%) | 71.4 | 66.7 | NA |
| CSs (%) | 16.7 | 20 | NA |
| HCQ (%) | 26.7 | 26.7 | NA |
| MTX (%) | 10 | NA | NA |
NA, not avaliable; CSs, corticosteroids; HCQ, hydroxychloroquine; MTX, methotrexate.
At week 28 (W28) 60% of the patients were considered to be responders and in the 86.7% persisted response at week 52 (W52). Two responders lost response from W28 to W52 because of increase in the pain VAS or in the fatigue VAS. Four patients, no responders at W28 but who had improved in at least one item of the composite primary end point, maintained the treatment until the end of study and 3/4 achieved response at W52.
Efficacy in drynessMariette et al.13 reported a reduced VAS dryness score at W28 in 37% of patients. The mean dryness VAS score changed from 7.8 (1.8) to 6.2 (2.9). De Vita et al.14 found a persistent response at W52, with no changes (versus W28) in the VAS dryness score.
No significant changes were reported in the Schirmer test, unstimulated whole salivary flow or in focus score of salivary gland biopsy.
Efficacy in systemic manifestationsPatients presented improvement in fatigue VAS score (26.7% at W28 and 60% at W52) and in pain VAS score (46.7% at W28 and 60% at W52) but this improvement was not statistically significant. Mariette et al.13 reported a significant difference in ESSPRI at W28 (mean score decreased from 6.4 (1.1) to 5.6 (2.0), p=0.0174), that persisted at W52.14
The disease activity was assessed through the ESSDAI. The mean ESSDAI score decreased significantly, mainly in glandular, articular, lymphadenopathy and biological domains at W28 and W52. The mean ESSDAI score decreased from 8.8 (7.4) at baseline to 6.3 (6.6) at W28 (p=0.0015) and at W52, in the 15 responders at W28, the ESSDAI was 3.1 (3.2) (p<0.0001 vs baseline). Quartuccio et al.15 reported, one year after the end of belimumab, an increase in the ESSDAI score to 7.0 (5.7) (p=0.2 vs baseline, p=0.003 vs W52).
Quality of life was evaluated through the SF-36 at W28 and W52 and no significant differences from baseline were found.
Efficacy in glandular involvementMarriette et al.13 demonstrated an improvement in non-malignant parotid swelling in 76.9% (10/13) of patients at W28, but parotid swelling did not improve in 2 patients with parotid low-grade lymphoma. This improvement persisted at W52 and worsened after the end of belimumab.
Efficacy in biological biomarkersThe trial authors found significant reduction of serum levels of immunoglobulins and levels of free light chains at W28. Moreover, the mean number of B cells decreased significantly. This improvement in B-cell biomarker values were maintained until W52. Moreover, there was a significant decrease in the mean rheumatoid factor (RF) titration at W28, which improved later at W52. Cryoglobulinaemia, present in three patients, cleared in all at W28.
SafetyThe most frequent adverse effects were headache and mild transient neutropenia. Only one serious adverse effect was reported at W28 (pneumococcus meningitis). Seventeen infections were described. The most frequent were upper respiratory tract infections followed by urinary tract infections and gastroenteritis. One patient developed breast cancer 3 monts after the last infusion. Also, one patient developed progressive scleroderma during follow-up. No infusion reactions occurred.
DiscussionThis present work summarized the published evidence evaluating the efficacy and safety of belimumab in patients with pSS. To date, there is lack of evidence to evaluate the efficacy of belimumab in pSS.
After this systematic literature review, only one open label study was retrieved, reporting its results in three different articles. Based on this weak evidence, belimumab13,14 was reported to have a moderate significant improvement in subjective symptoms like dryness and patients perception, despite the small number evaluated.
However, the greatest improvement was found in some objective parameters, significantly and persistently reducing systemic activity, glandular involvement and biological biomarkers. Non-malignant parotid enlargement improved in most patients and cryoglobulinaemia disappeared in all of them. Taking into account that almost a 10% of pSS patients are estimated to develop a B-cell lymphoma16 and the major risk factors for lymphoma development are systemic activity, cryoglobulinaemia, RF, lymphadenopathy and non-neoplastic salivary gland enlargement, these findings should be highlighted considering their relevance in lymphoproliferation in pSS.17–19
The most common adverse events with belimumab were viral upper respiratory tract infections, mild transient neutropenia and headache at the end of infusion. Treatment was well tolerated without infusional reactions. The safety profile was generally consistent with that previously observed in belimumab trials for the treatment of SLE.9,20
Nevertheless, the results of this systematic literature review present important limitations. First, we only found one clinical trial (results evaluated at W28, W52 and at the end of trial), with a small sample and without control group, nor randomization. Secondly, the quality of evidence for this study is low (LE 3). In addition, pSS is a heterogeneous autoimmune disease with variable organ involvement, therefore it is difficult to identify which patients will respond and benefit from biological treatment.
Actually, a multicenter, double-blind, placebo-controlled trial is being carried out to evaluate whether treatment with belimumab monotherapy or belimumab/rituximab co-administration has a substantive effect on disease activity, but results are not available yet.
To sum up, the published evidence to determine the efficacy of belimumab in pSS to date, is sadly limited. Belimumab seems to be effective in the decrease of the systemic activity, parotid enlargement, lymphadenopathies, articular manifestations and B cell biomarkers. Nevertheless, to face the scarcity, more data are required to confirm this trend.
FundingThis project was funded by the Spanish Society of Rheumatology.
Conflict of interestDr. Corominas has recently received grant/research support from and/or provided board expert advice to: AbbVie, Amgen, AstraZeneca, Lilly, MSD, BMS, Janssen Pharmaceuticals, Novartis-Sandoz, Pfizer and Sanofi.
Dr. Andreu has recently received grant/research support from consultancy, speaker and/or research grants to: Abbvie, Gebro, MSD, Pfizer, Antares, GSK, Novartis-Sandoz, Sanofi, UCB, AstraZeneca, Biogen and Celltrion.
Dr. Navarro-Compán has recently received grant/research support from consultancy, speaker and/or research grants to: Abbvie, BMS, Lilly, MSD, Novartis, Pfizer, Roche and UCB.
The other authors have declared no conflicts of interest.