The etiopathogenesis of ankylosing spondylitis (AS), which is a chronic, progressive, inflammatory, systemic disease, has not been fully elucidated yet. Thiol-disulfide homeostasis, a component of antioxidant defense, is thought to play a role in the etiology of inflammatory diseases. We aimed to evaluate the existence of oxidative stress in active AS patients with thiol-disulfide homeostasis.
Materials and methodsPatients who were found to have high (n: 27) and very-high (n: 18) activity levels with ASDAS-ESR and 40 healthy controls participated in the study. Serum native-thiol (NT), total-thiol (TT), and disulfide levels were analyzed by an automated colorimetric method.
ResultsWhile TT and NT levels were significantly decreased in patients compared to the control group, the disulfide levels were increased. There was a significant negative correlation between ESR, and NT, TT in both groups and also between hsCRP and NT, TT in very-high active AS patients.TT and NT levels were significantly higher in the nonsteroidal anti-inflammatory drug (NSAID) users compared to those using biological agents.
ConclusionsThe deterioration of thiol-disulfide homeostasis in favor of disulfide; correlations between ESR, CRP, and NT, TT support the use of thiol-disulfide variables in determining the disease activity level.
La etiopatogenia de la espondilitis anquilosante (EA), que es una enfermedad crónica, progresiva, inflamatoria y sistémica, aún no se ha dilucidado por completo. Se cree que la homeostasis del tiol-disulfuro, un componente de la defensa antioxidante, desempeña un papel en la etiología de las enfermedades inflamatorias. Nuestro objetivo fue evaluar la existencia de estrés oxidativo en pacientes con EA activa con homeostasis de tiol-disulfuro.
Materiales y métodosParticiparon en el estudio pacientes que tenían niveles de actividad altos (n: 27) y muy altos (n: 18) con ASDAS-ESR y 40 controles sanos. Los niveles séricos de tiol nativo (NT), tiol total (TT) y disulfuro se analizaron mediante un método colorimétrico automático.
ResultadosSi bien los niveles de TT y NT disminuyeron significativamente en los pacientes en comparación con el grupo de control, los de disulfuro aumentaron. Hubo una correlación negativa significativa entre ESR y NT, TT en ambos grupos y también entre hsCRP y NT, TT en pacientes con EA muy alta activa. Los niveles de TT y NT fueron significativamente más altos en los usuarios de medicamentos antiinflamatorios no esteroideos (AINE) en comparación con los que utilizan agentes biológicos.
ConclusionesEl deterioro de la homeostasis tiol-disulfuro a favor del disulfuro y las correlaciones entre ESR, CRP y NT, TT apoyan el uso de variables de tiol-disulfuro para determinar el nivel de actividad de la enfermedad.
Ankylosing spondylitis (AS), which is a chronic, systemic, and inflammatory disease, can affect axial joints, spine, enthesis areas, sacroiliac joints and peripheral joints.1,2 Its etiopathogenesis has not been fully elucidated. Although its prevalence varies between countries (0.007–2%), it is relatively high in societies where the HLA-B27 positivity rate is high (like Caucasian population).3,4 If left untreated, it negatively affects the quality of life by causing physical and functional damage in the long term.1 Although inflammatory markers (such as CRP, ESR) are used in diagnosis and follow-up, they are often limited in terms of assessing disease activity level and progression. Questionnaires are used to evaluate conditions such as disease activity and functional limitations due to insufficient laboratory tests. The Ankylosing Spondylitis Disease Activity Score (ASDAS) is one of the methods used for determining disease activity status. ASDAS is a disease activity index calculated by using clinical findings such as low back pain, morning stiffness, peripheral joint pain/swelling, global assessment of the patient, and ESR or CRP values. According to ASDAS patient disease activity status is defined as inactive disease (<1.3), moderate (1.3–2.1), high (2.1–3.5) and very high (>3.5) disease activity. The three cut-offs selected to separate these states are: 1.3, 2.1 and 3.5 units.2
There is a delicate balance between the oxidative–antioxidative system. It has been shown that oxidative stress, which is characterized by increased reactive oxygen species (ROS) or insufficient antioxidant defense, is associated with the pathogenesis of some inflammatory diseases, and increased neutrophil activity in patients with AS causes excessive production of reactive oxygen species.
Thiols are organic molecules containing a sulfhydryl group (–SH). Low molecular weight molecules such as glutathione, cysteine, homocysteine, cysteinylglycine and gamma-glutamylcysteine and proteins such as albumin constitute the plasma thiol pool and play an important role in maintaining the oxidant–antioxidant balance of the body. The sulfhydryl groups of thiols react reversibly with oxidants to form disulfide bridges, which are covalent bonds. Thiol/disulfide homeostasis, which is an important part of the antioxidant defense system, has important roles in processes such as apoptosis, regulation of enzymatic reactions and signal pathways, and detoxification. It has been shown that thiol/disulfide homeostasis is disturbed in various inflammatory diseases.5–8
In this study, we aimed to evaluate whether there is a change in the thiol-disulfide homeostasis by analyzing serum native thiol (NT), total thiol (TT), and disulfide levels by the automated colorimetric method in AS patients with high and very high disease activity.
Materials and methodsStudy group and designIn the present study, 45 active AS patients who were diagnosed according to the modified New York diagnostic criteria9 and whose activity score was above 2.1 according to the Ankylosing Spondylitis Disease Activity Score-Erythrocyte Sedimentation Rate (ASDAS-ESR)2 and 40 healthy age, gender, and body mass index matched individuals were included. Alcohol and cigarette users, patients who have hypertension, diabetes mellitus, obesity, hyperlipidemia were not included in the study. 5mL fasting venous blood was obtained from the patients who signed the informed consent form. After the blood was centrifuged at 2000g for 10min, their serum was separated. Samples were stored at −80°C until the biochemical analysis. Demographic characteristics, laboratory results of the groups, and scores related to disease activity were recorded. The study was conducted in compliance with the Helsinki declaration, all of the participants gave their informed consent and ethical approval was taken from the ethics committee of Recep Tayyip Erdogan University (no. 43/23.12.2016).
Biochemical analysisTT, NT, disulfide levels of the samples were measured by the method applied using the modified Ellman reagent developed by Erel et al.5 This method is based on the reduction of disulfide bonds to reactive thiol groups. After serum NT, TT, and disulfide levels were analyzed, disulfide/native thiol (DNT), disulfide/total thiol (DTT), native thiol/total thiol (NTT) ratios were calculated for all individuals.
Statistical analysisStatistical analysis was performed using SPSS version 21. Whether the variables conformed to normal distribution were examined using histogram graphics and Shapiro–Wilk test. Normally distributed continuous variables were compared between groups using one-way ANOVA analysis of variance test. Pairwise comparisons were evaluated as significance level p<0.017 after post hoc Bonferroni correction tests when significant differences were found between overall groups. The Levene test was used to evaluate the homogeneity of variances. Variables that did not conform to normal distribution were compared using the Kruskal–Wallis test. In cases where there was a difference, pairwise comparisons were made using the Mann–Whitney U test and evaluated using the Bonferroni correction. The relationship between variables was evaluated using the Pearson and Spearman tests according to the distribution of the variables. The statistical significance level was accepted as p<0.05. The distribution of categorical variables among groups was evaluated by Chi-square analysis. Independent predictors were analyzed with multivariate logistic regression analysis. Variables that included to multivariate regression model were acquired as a result of univariate regression analysis as p<0.2. Ultimate multivariate logistic regression model that considered sample size and evaluated with Hosmer–Lemeshow goodness of fit statistics was conducted through enter method.
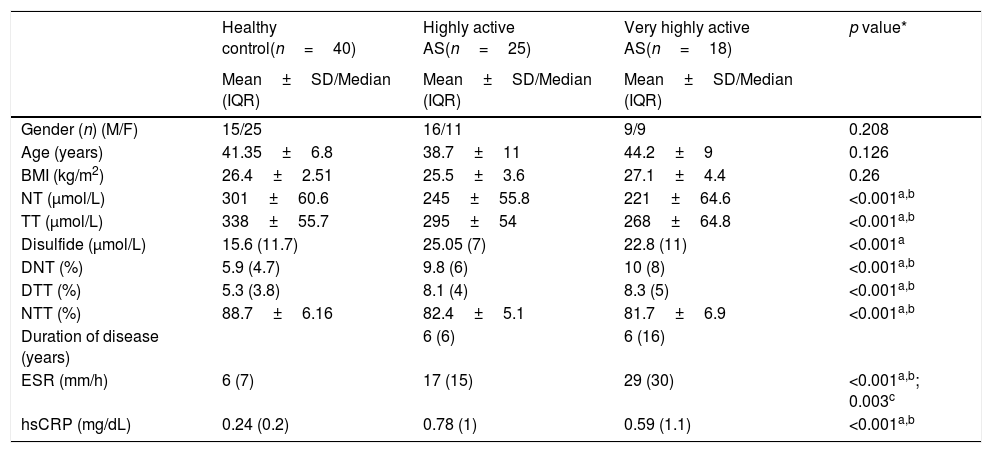
ResultsData on age, gender, BMI, duration of disease, and other laboratory results of the active AS patient and control groups are presented in Table 1. There was no statistically significant difference between the age, gender, and BMI variables of the groups (p values 0.126, 0.208, 0.26 respectively). NT, TT levels were decreased significantly in highly and very highly active AS patients compared to the control group. Disulfide level were significantly increased in highly active AS group compared to control group. An increase in disulfide levels was observed in very highly active group compared to control but it was not significant (p=0.023).
Demographic characteristics and laboratory results of healthy control group, highly and very highly active AS patients.
| Healthy control(n=40) | Highly active AS(n=25) | Very highly active AS(n=18) | p value* | |
|---|---|---|---|---|
| Mean±SD/Median (IQR) | Mean±SD/Median (IQR) | Mean±SD/Median (IQR) | ||
| Gender (n) (M/F) | 15/25 | 16/11 | 9/9 | 0.208 |
| Age (years) | 41.35±6.8 | 38.7±11 | 44.2±9 | 0.126 |
| BMI (kg/m2) | 26.4±2.51 | 25.5±3.6 | 27.1±4.4 | 0.26 |
| NT (μmol/L) | 301±60.6 | 245±55.8 | 221±64.6 | <0.001a,b |
| TT (μmol/L) | 338±55.7 | 295±54 | 268±64.8 | <0.001a,b |
| Disulfide (μmol/L) | 15.6 (11.7) | 25.05 (7) | 22.8 (11) | <0.001a |
| DNT (%) | 5.9 (4.7) | 9.8 (6) | 10 (8) | <0.001a,b |
| DTT (%) | 5.3 (3.8) | 8.1 (4) | 8.3 (5) | <0.001a,b |
| NTT (%) | 88.7±6.16 | 82.4±5.1 | 81.7±6.9 | <0.001a,b |
| Duration of disease (years) | 6 (6) | 6 (16) | ||
| ESR (mm/h) | 6 (7) | 17 (15) | 29 (30) | <0.001a,b; 0.003c |
| hsCRP (mg/dL) | 0.24 (0.2) | 0.78 (1) | 0.59 (1.1) | <0.001a,b |
BMI: body mass index; NT: native thiol; TT: total thiol; DNT: disulfide/native thiol rate; DTT: disulfide/total thiol rate; NTT: native thiol/total thiol rate; ESR: erythrocyte sedimentation rate; hsCRP: high sensitivity C-reactive protein; SD: standard deviation; IQR: interquartile range; M: male; F: female.
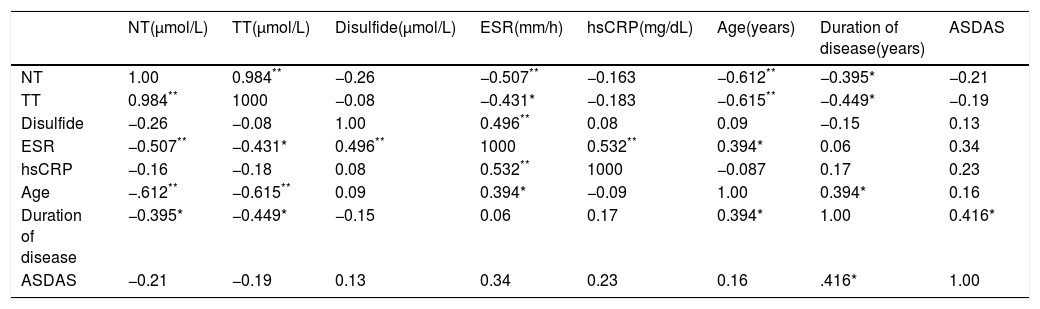
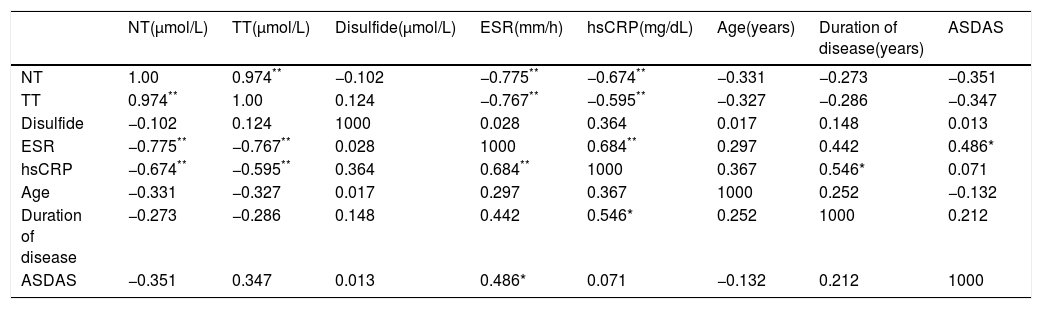
The correlation coefficients of variables in highly and very highly active AS patients is given in Tables 2 and 3.
Correlation analysis between thiol/disulfide variables and other parameters in highly active AS patients.
| NT(μmol/L) | TT(μmol/L) | Disulfide(μmol/L) | ESR(mm/h) | hsCRP(mg/dL) | Age(years) | Duration of disease(years) | ASDAS | |
|---|---|---|---|---|---|---|---|---|
| NT | 1.00 | 0.984** | −0.26 | −0.507** | −0.163 | −0.612** | −0.395* | −0.21 |
| TT | 0.984** | 1000 | −0.08 | −0.431* | −0.183 | −0.615** | −0.449* | −0.19 |
| Disulfide | −0.26 | −0.08 | 1.00 | 0.496** | 0.08 | 0.09 | −0.15 | 0.13 |
| ESR | −0.507** | −0.431* | 0.496** | 1000 | 0.532** | 0.394* | 0.06 | 0.34 |
| hsCRP | −0.16 | −0.18 | 0.08 | 0.532** | 1000 | −0.087 | 0.17 | 0.23 |
| Age | −.612** | −0.615** | 0.09 | 0.394* | −0.09 | 1.00 | 0.394* | 0.16 |
| Duration of disease | −0.395* | −0.449* | −0.15 | 0.06 | 0.17 | 0.394* | 1.00 | 0.416* |
| ASDAS | −0.21 | −0.19 | 0.13 | 0.34 | 0.23 | 0.16 | .416* | 1.00 |
NT: native thiol; TT: total thiol; ESR: erythrocyte sedimentation rate; hsCRP: high sensitivity C-reactive protein; ASDAS: Ankylosing Spondylitis Disease Activity Score.
Correlation analysis between thiol/disulfide variables and other parameters in very highly active AS patients.
| NT(μmol/L) | TT(μmol/L) | Disulfide(μmol/L) | ESR(mm/h) | hsCRP(mg/dL) | Age(years) | Duration of disease(years) | ASDAS | |
|---|---|---|---|---|---|---|---|---|
| NT | 1.00 | 0.974** | −0.102 | −0.775** | −0.674** | −0.331 | −0.273 | −0.351 |
| TT | 0.974** | 1.00 | 0.124 | −0.767** | −0.595** | −0.327 | −0.286 | −0.347 |
| Disulfide | −0.102 | 0.124 | 1000 | 0.028 | 0.364 | 0.017 | 0.148 | 0.013 |
| ESR | −0.775** | −0.767** | 0.028 | 1000 | 0.684** | 0.297 | 0.442 | 0.486* |
| hsCRP | −0.674** | −0.595** | 0.364 | 0.684** | 1000 | 0.367 | 0.546* | 0.071 |
| Age | −0.331 | −0.327 | 0.017 | 0.297 | 0.367 | 1000 | 0.252 | −0.132 |
| Duration of disease | −0.273 | −0.286 | 0.148 | 0.442 | 0.546* | 0.252 | 1000 | 0.212 |
| ASDAS | −0.351 | 0.347 | 0.013 | 0.486* | 0.071 | −0.132 | 0.212 | 1000 |
NT: native thiol; TT: total thiol; ESR: erythrocyte sedimentation rate; hsCRP: high sensitivity C-reactive protein; ASDAS: Ankylosing Spondylitis Disease Activity Score.
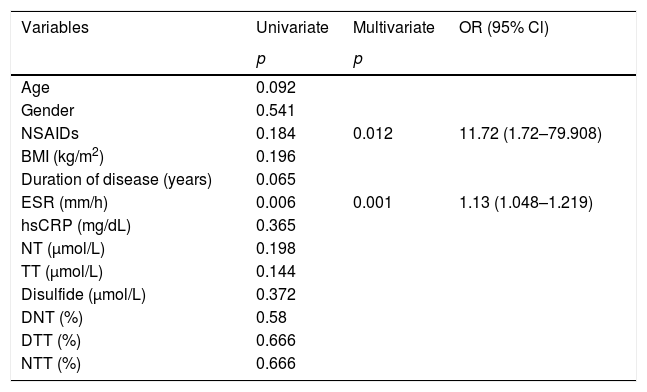
The power of variables to predict very high activity level individuals in the AS patient group was evaluated by multivariate logistic regression analysis. Drug group and ESR were found as independent factors associated with very high disease activity level. The risk of developing the very high activity disease in NSAIDs group was higher 11.72 times than biologic agent group, and with a unit increase in ESR the risk of developing the very high activity disease increases by 1.13 times according to the constructed multivariate logistic regression model. Model fit was assessed with the Hosmer and Lemeshow goodness of fit test. And the model was found suitable with p=0.222 (p>0.05) (Table 4).
Independent factors associated with very high disease activity level in binary logistic regression model.
| Variables | Univariate | Multivariate | OR (95% Cl) |
|---|---|---|---|
| p | p | ||
| Age | 0.092 | ||
| Gender | 0.541 | ||
| NSAIDs | 0.184 | 0.012 | 11.72 (1.72–79.908) |
| BMI (kg/m2) | 0.196 | ||
| Duration of disease (years) | 0.065 | ||
| ESR (mm/h) | 0.006 | 0.001 | 1.13 (1.048–1.219) |
| hsCRP (mg/dL) | 0.365 | ||
| NT (μmol/L) | 0.198 | ||
| TT (μmol/L) | 0.144 | ||
| Disulfide (μmol/L) | 0.372 | ||
| DNT (%) | 0.58 | ||
| DTT (%) | 0.666 | ||
| NTT (%) | 0.666 |
In the multivariate regression model drug group and ESR were included as covariates. Statistical significance was assumed at p<0.05. Hosmer and Lemeshov test p=0.222, Nagelkerke R Square=0.489.
Cl: confidence interval; OR: odds ratio; BMI: body mass index; ESR: erythrocyte sedimentation rate; hsCRP: high sensitivity C-reactive protein; NT: native thiol; TT: total thiol; DNT: disulfide/native thiol rate; DTT: disulfide/total thiol rate; NTT: native thiol/total thiol rate.
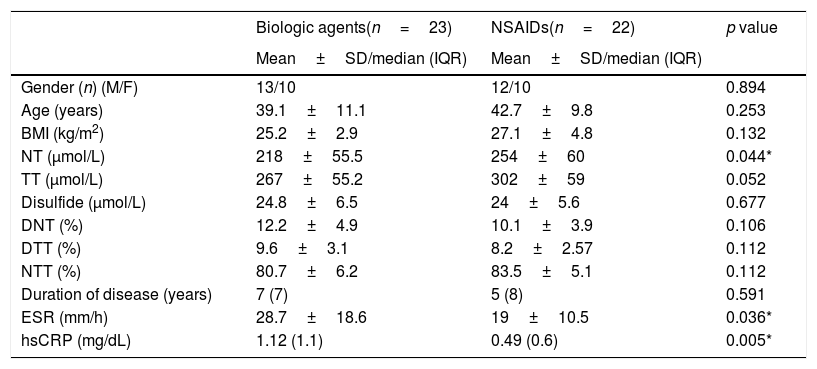
While 22 patients were receiving NSAIDs, the other 23 patients were receiving biological agents (infliximab, adalimumab, golimumab, etanercept). There was no significant difference between the drug groups used in patients with high and very high activity levels (p=0.181). After drugs were divided into two groups as NSAID and biological agents, we observed that TT (p=0.052) and NT (p=0.044) levels were higher in the group receiving NSAIDs (Table 5). After bootstrapping (1000 bootstrap) for TT and NT variables p values were became 0.049 and 0.033 respectively. Sample numbers were insufficient for subgroup analysis in patients using biological agents.
Demographic characteristics and laboratory results according to drug groups.
| Biologic agents(n=23) | NSAIDs(n=22) | p value | |
|---|---|---|---|
| Mean±SD/median (IQR) | Mean±SD/median (IQR) | ||
| Gender (n) (M/F) | 13/10 | 12/10 | 0.894 |
| Age (years) | 39.1±11.1 | 42.7±9.8 | 0.253 |
| BMI (kg/m2) | 25.2±2.9 | 27.1±4.8 | 0.132 |
| NT (μmol/L) | 218±55.5 | 254±60 | 0.044* |
| TT (μmol/L) | 267±55.2 | 302±59 | 0.052 |
| Disulfide (μmol/L) | 24.8±6.5 | 24±5.6 | 0.677 |
| DNT (%) | 12.2±4.9 | 10.1±3.9 | 0.106 |
| DTT (%) | 9.6±3.1 | 8.2±2.57 | 0.112 |
| NTT (%) | 80.7±6.2 | 83.5±5.1 | 0.112 |
| Duration of disease (years) | 7 (7) | 5 (8) | 0.591 |
| ESR (mm/h) | 28.7±18.6 | 19±10.5 | 0.036* |
| hsCRP (mg/dL) | 1.12 (1.1) | 0.49 (0.6) | 0.005* |
BMI: body mass index; NT: native thiol; TT: total thiol; DNT: disulfide/native thiol rate; DTT: disulfide/total thiol rate; NTT: native thiol/total thiol rate; ESR: erythrocyte sedimentation rate; hsCRP: high sensitivity C-reactive protein; SD: standard deviation: IQR: interquartile range; M: male; F: female.
Thiol-disulfide homeostasis, which is one of the important components of antioxidant defense, has important roles in cases such as apoptosis, regulation of enzyme activities, and detoxification.5,10 Numerous studies have revealed that in the presence of inflammatory and degenerative diseases, malignancies, the thiol-disulfide homeostasis may be impaired.11–15 Although the pathogenesis has not been fully revealed, it is thought that reactive oxygen species contribute to the disease process as well as autoimmune, genetic, and inflammation mechanisms.
Ozgocmen et al.16 reported that oxidant–antioxidant balance was impaired in favor of oxidant system (decrease in catalase enzyme levels, increase in MDA levels) in patients with AS, especially in patients with active disease. And there was no significant difference between inactive AS patients and healthy control patients in view of nitric oxide level, catalase, superoxide dismutase activity, and malondialdehyde levels. Similarly we found an increase for disulfide in highly and very highly AS group in our study.
Although in various studies, it has been shown that oxidative stress related parameters decreased in patients with AS compared to the healthy control group.17 However, there are studies with contradictory results.18 This discrepancy may be due to the difference in duration of illness, disease activity grade, concomitant diseases and therapies implemented. However, these findings clearly show that the oxidant–antioxidant balance is impaired in AS patients. Thiol-disulfide homeostasis plays an important role in the antioxidant defense system. Various studies have shown that thiol-disulfide homeostasis plays a protective role against ROS-induced cell damage.19,20
Our results that TT and NT levels were decreased in highly active AS patients are consistent with the study carried out by Dogru et al.21 They also reported DNT, DTT, NTT levels in inactive patients were similar to the control group. Furthermore we evaluated active AS patients under two subgroups as highly active and very highly active in our study, and found no statistical significant difference between thiol-disulfide homeostasis parameters, but ESR.
Baykara et al.22 reported that NT, NTT levels were significantly decreased in AS patients; disulfide DNT and DTT levels increased significantly and these changes were also associated with duration of disease. They reported a negative correlation between CRP and NT values, and a positive correlation between CRP and disulfide values. Likewise according to our findings ESR and hsCRP were negative correlated with NT and TT.
Studies support that thiol-disulfide homeostasis is markedly impaired in AS patients. In our study, we observed that the thiol-disulfide homeostasis in patients with high and very high disease activity changed in favor of disulfide like other studies (decrease in TT and NT levels, increase in disulfide level). Besides, while other studies have low level or no correlation between ESR, CRP, and NT, TT, in our study, we found moderate or high correlation levels. These findings may also support that thiol-disulfide homeostasis is significantly affected in active AS patients and impaired balance may be related to disease severity.
Numerous studies have been reported about the effects of NSAIDs on oxidative stress.23–25 Although various mechanisms have been suggested, the mechanism underlying the oxidant and antioxidant effects of NSAIDs has not been resolved, yet. In our study, an increase was found in NT and TT levels in the NSAID group. Thiol-disulfide homeostasis may mediate the antioxidant effects of NSAIDs. There are studies in the literature showing that biological agents such as infliximab have antioxidant effects as well as anti-inflammatory effects.26,27 Although we found higher TT and NT levels in patients in the NSAIDs group, not knowing the duration of drug use of the patients and the insufficient number of patients in biological agent subgroups for evaluation can be considered among the shortcomings of our study. The small number of patients in the groups and implementation of ASDAS-ESR scoring instead of ASDAS-CRP in our research can be thought of as limitations. Although ASDAS-CRP is frequently used in similar studies, we preferred to use ASDAS-ESR because of its good correlation with ASDAS-CRP. Besides, the doses and duration of the medication used by our patients were not recorded, as this was not the focus of our study. It can be considered as another limitation.
However, studies in the literature was not enough to show that thiol-disulfide homeostasis changed in favor of disulfide and data related to oxidative stress were contradictory. The changes in TT, NT, and disulfide levels of AS patients in different publications were not compatible with each other.21,22 The heterogeneity of the groups in terms of implemented treatments, age, activity level, and also the fact that the tests used to determine the activity level are mostly based on subjective patient data can be considered the reasons for these conflicting results. Instead of the BASDAI score, which is based entirely on the verbal reporting of the patients we used ASDAS, which also includes objective laboratory measurements like ESR and thought to be superior than BASDAI in specifying disease activity level.28,29
We thought that patients with inactive and moderate disease activity could cause these contradictory results. The shift of thiol-disulfide homeostasis in favor of disulfide in the high and very high activity patient group supported our hypothesis. This shift, correlations between ESR, CRP, and TT, NT variables suggest that thiol-disulfide homeostasis may be useful in identifying patients with high activity levels and using supportive treatment strategies such as antioxidants.
One of the most significant deficiencies of studies on oxidant–antioxidant levels is the lack of reference range and cut-off values that can be used for assessing diseases. In a study, the reference range for the disulfide level was found to be 6.65–27.93μmol/L.5 Considering these values, 29/45 of our patients were within the reference range in terms of disulfide levels, while 10/40 of our control group were outside of the reference range. This can be considered as a complicating factor in evaluating the results. In order to use these variables in the diagnosis and follow-up of diseases, it is necessary to conduct studies with broad participation and standardized groups that reference intervals and cut-off values can be determined.
ConclusionsTo summarize, the deterioration of thiol-disulfide homeostasis in favor of disulfide; correlations between ESR, CRP, and NT, TT support the use of thiol-disulfide variables in determining the disease activity level thus thiol-disulfide homeostasis could contribute to the clarification of the pathogenesis of AS. Finally it can be concluded that thiol-disulfide homeostasis may be useful in elucidating the antioxidant action mechanisms of NSAIDs.
Conflict of interestThe authors declare no conflict of interest in this study.