We aimed to develop recommendations for the management of methotrexate (MTX) when considering the combination with biological (b) or targeted synthetic (ts) disease modifying drugs (DMARDs) in rheumatoid arthritis (RA).
MethodsEleven experts on RA were selected. Two coordinators formulated 13 questions about the combination therapy of MTX with bDMARDs or tsDMARDs. A systematic review was conducted to answer the questions. Inclusion and exclusion criteria were established as well as the search strategies (Medline, Embase and the Cochrane Library were searched up to January 2019). Two reviewers selected the articles and collected data. Simultaneously, EULAR and ACR meeting abstracts were evaluated. Based on this evidence, the coordinators proposed preliminary recommendations that the experts discussed and voted in a nominal group meeting. The level of evidence and grade of recommendation was established using the Oxford Center for Evidence Based Medicine and the level of agreement with a Delphi. Agreement was established if at least 80% of the experts voted ‘yes’ (yes/no).
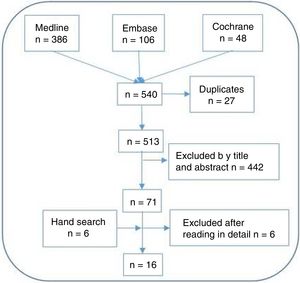
ResultsThe systematic review retrieved 513 citations of which 61 were finally included. A total of 10 recommendations were generated, voted and accepted. The level of agreement was very high in all of them and it was achieved in the first Delphi round. Final recommendations cover aspects such as the optimal MTX dosage, tapering strategy or patients’ risk management.
ConclusionsThis document is intended to help clinicians solve usual clinical questions and facilitate decision making when treating RA patients with MTX in combination with bDMARDs or tsDMARDs.
Desarrollar recomendaciones sobre el uso de metotrexato (MTX) en combinación con medicamentos modificadores de la enfermedad (DMARD) biológicos (b) o sintéticos específicos (ts) en la artritis reumatoide (AR).
MétodosSe seleccionaron 11 expertos en AR. Dos coordinadores formularon 13 preguntas sobre la terapia combinada de MTX con bDMARD o tsDMARD. Se realizó una revisión sistemática para responder las preguntas. Se establecieron criterios de inclusión y exclusión, así como las estrategias de búsqueda (se realizaron búsquedas en Medline, Embase y la Biblioteca Cochrane hasta enero de 2019). Dos revisores seleccionaron los artículos y recopilaron datos. Simultáneamente, se evaluaron los resúmenes de las reuniones EULAR y ACR. Con base en esta evidencia, los coordinadores propusieron recomendaciones preliminares que los expertos discutieron y votaron en una reunión de grupo nominal. El nivel de evidencia y el grado de recomendación se establecieron utilizando el Centro de Oxford para Medicina Basada en Evidencia y el nivel de acuerdo con un Delphi. El acuerdo se estableció si al menos el 80% de los expertos votaron «sí» (sí/no).
ResultadosLa revisión sistemática recuperó 513 citas, de las cuales finalmente se incluyeron 61. Se generaron, votaron y aceptaron un total de 10 recomendaciones. El nivel de acuerdo fue muy alto en todas ellas y se logró en la primera ronda de Delphi. Las recomendaciones finales cubren aspectos como la dosis óptima de MTX, la estrategia de reducción o la gestión del riesgo de los pacientes.
ConclusionesEste documento está destinado a ayudar a los médicos a resolver preguntas clínicas habituales y facilitar la toma de decisiones al tratar a pacientes con AR con MTX, en combinación con bDMARD o tsDMARD.
Methotrexate (MTX) is the anchor in the treatment of rheumatoid arthritis (RA) and the most commonly prescribed conventional synthetic disease modifying anti-rheumatic drug (csDMARD), either as monotherapy or in combination with biologic DMARDs (bDMARDs) or targeted synthetic DMARDs (tsDMARD).1–4
Current European League Against Rheumatism (EULAR) recommendations for the treatment of RA1 support the use of MTX plus short term glucocorticoids as a first-line treatment, with the aim of achieving a>50% improvement within 3 months and clinical remission within 6 months.1 If this strategy fails, due to inadequate response, stratification based on disease prognosis is recommended. In the absence of unfavorable prognostic markers (autoantibodies, high disease activity, early erosions, failure of 2 csDMARDs), patients should switch to or add another csDMARD. But, if unfavorable prognostic markers are present, or if the previous step has failed, a bDMARD or tsDMARD should be added to the csDMARD.1 Other Spanish and American national initiatives are also in line with EULAR recommendations.2–4
Although these documents cover different aspects of the use of MTX in combination with b- or tsDMARD,1–4 some clinical questions that are important in daily practice remain unclear. These questions include, for example, what are the optimal doses and route of administration of MTX at the start of the combination therapy, or the tapering strategy in patients that have achieved the therapeutic target.
Bearing in mind all previous considerations, the aim of this project was to provide specific and practice guide regarding the use of MTX combination therapy with bDMARDs o tsDMARDs, based on the best evidence and experts opinion. We are confident that these recommendations will help health professionals involved in the management of RA patients.
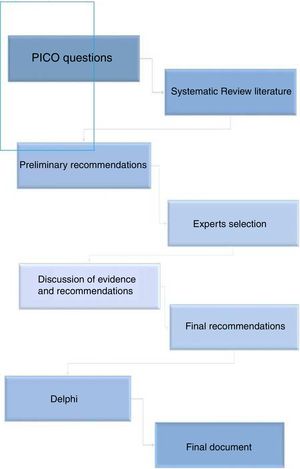
MethodsStudy design and panel selectionThe consensus statement has been developed using nominal group and Delphi techniques, along with a systematic literature review (SLR). The project was carried out following the Declaration of Helsinki ethical principles for medical research involving human subjects, and in accordance with the Good Clinical Practice regulations. First, a group of 11rheumatologists (2 of them the coordinators) with interest and demonstrated experience in the management of RA were designated (Fig. 1).
Systematic literature reviewThe coordinators defined 13 questions (Table 1), connected to the use of MTX in combination with b- or tsDMARDs, that included the indication of the combination therapy, MTX dose, route of administration, dose adjustments, etc. A comprehensive SLR was performed to address these questions. The following PICO queries and inclusion criteria were defined: (1) Adult RA patients (population); (2) on, or considering the start of combined therapy with MTX and b-or tsDMARDs approved for RA including 5 tumor necrosis factor (TNF) inhibitors (infliximab, adalimumab, etanercept, golimumab, certolizumab pegol), abatacept (ABT), rituximab (RTX), interleukin 6 (IL-6) inhibitors tocilizumab and sarilumab, biosimilars, and Janus kinase inhibitors (JAKs) tofacitinib and baricitinib (intervention); (3) compared with placebo or an active drug (comparator); reporting efficacy and/or safety variables like composite activity indexes, radiographic progression, serious adverse events, etc. (outcomes); (4) searches were restricted to SLR and meta-analysis based on randomized controlled trials (RCTs) (study design), humans, and articles written in English and/or Spanish. The publications were identified by sensitive search strategies in the main bibliographic databases. For this purpose, an expert librarian designed the search strategies, using Mesh and text word terms. The following bibliographic databases were screened up to January 2019: Medline, Embase and the Cochrane Library. The abstracts of the two previous (2017 and 2018) annual scientific meetings of the American College of Rheumatology (ACR) and EULAR were also examined along with national and international consensus and guidelines. Two reviewers selected the articles and collected data, independently. Subsequently, a manual search of the bibliography of the articles that were finally included was performed. The quality was evaluated with the Oxford Center for Evidence Based Medicine recommendations.5 Subsequently, the coordinators proposed a set of preliminary recommendations based on the SLR results.
Research questions regarding the use of methotrexate in combination with biologic and targeted synthetic disease modifying drugs in rheumatoid arthritis.
| # | Questions |
|---|---|
| 1 | In patients with active RA and inadequate response to MTX, when adding a bDMARD, is it better to continue or to stop MTX? |
| 2 | In patients with active RA and inadequate response to MTX, when adding a JAK inhibitor, is it better to continue or to stop MTX? |
| 3 | Which is the optimal MTX dose when combined with bDMARD? |
| 4 | Which is the optimal MTX dose when combined with JAKs inhibitors? |
| 5 | In patients who have achieved and maintained the treatment goal: is it better to taper MTX or the bDMARD? |
| 6 | In patients who have achieved and maintained the treatment goal: is it better to taper MTX or the JAK-inhibitor? |
| 7 | Is MTX the csDMARD of choice to combine with bDMARDs (regarding efficacy, safety and survival)? |
| 8 | Is MTX the csDMARD of choice to combine with JAK-inhibitors (regarding efficacy, safety and survival)? |
| 9 | Does MTX in combination with bDMADRS/JAKs inhibitors increase these drugs survival? |
| 10 | Which is MTX influence on bDMADRS/JAKs inhibitors immunogenicity when combined? |
| 11 | Which is parenteral MTX role when combined with bDMADRS/JAKs inhibitors? |
| 12 | Is the combination of MTX with bDMADRS/JAKs inhibitors safe? |
| 13 | Is risk management of MTX different when combined with bDMARDS/Jak inhbitors? |
Abbreviations: MTX=methotrexate; RA=rheumatoid arthritis; JAKs=Januskinases; bDMARDs=biological disease modifying drugs; csDMARDs=conventional synthetic disease modifying drugs; bDMARDs=biological disease modifying drugs.
The experts held a nominal meeting in which objectives, scope and users were defined. Then, through guided discussions, the results of the SLR and the preliminary recommendations were analyzed. Afterwards, definitive recommendations were generated.
DelphiRecommendations were submitted to a Delphi process. All experts voted ‘yes’ or ‘no’ for every recommendation. Agreement was defined when at least 80% of experts voted ‘yes’. This high cut-off was established because the coordinators wanted to achieve a maximum level of agreement among the experts (when possible). Recommendations with a grade of agreement (GA) inferior to 80% were reassessed and, if appropriate, re-edited and voted on in a second round.
Final consensus documentAfter the Delphi, and along with the results of the SLR, the final document was written. For each recommendation, the level of evidence (LE) and grade of recommendation (GR) were assigned according to the recommendations of the Oxford Center for Evidence Based Medicine recommendations.5
ResultsSystematic literature reviewThe SLR retrieved 530 articles (see Fig. 2). After the selection processes and the hand search 61 studies were finally included.
Recommendations, evidence and expert's considerations
A total of 9 preliminary recommendations were proposed: 2 were not voted and were explained in the main text of the document and 3 new ones were generated. Finally, 10 recommendations were voted and accepted. The level of agreement was very high in all of them and was achieved in the first Delphi round. Table 2 depicts the recommendations with their respective LE, GR and GA.
Recommendations with their level of evidence (LE), grade of recommendation (GR) and grade ofagreement (GA).
| # | Recommendation | LE | GR | GA |
|---|---|---|---|---|
| 1 | In patients with active RA and inadequate response to MTX, this drug should be continued when starting a TNF inhibitor (LE 1a; GR A), ABT (LE 2a; GR B), RTX (LE 1b-2a; GR B) | – | – | 100% |
| 2 | MTX should not be discontinued in patients with active RA and inadequate response to MTX who start IL-6 inhibitors | 1a | A | 91% |
| 3 | MTX should not be discontinued in patients with active RA and inadequate response to MTX who start JAKs inhibitors | 1b | A | 91% |
| 4 | When starting a combined therapy with MTX and bDMARDs in MTX-inadequate responders RA patients, it is recommended to continue with the same MTX doses | 1b | A | 100% |
| 5 | When combining MTX with TNF inhibitors, the dose of MTX should be at least 10mg/week | 1b | A | 100% |
| 6 | When starting a combined therapy with MTX and JAKs inhibitorsin MTX inadequate responders RA patients, it is recommended to continue with the same MTX doses | 1b | A | 100% |
| 7 | In RA patients who have achieved and sustained the treatment goal, the panel recommends, as treatment strategy, to give priority to the de-escalation of the bDMARDs, which does not exclude MTX dose adjustments or even MTX withdrawal in some intolerant patients on IL-6 inhibitors | 5 | D | 100% |
| 8 | bDMARDs should be combined with MTX as the first csDMARD choice, although other csDMARDs could be considered in case of MTX intolerance/contraindication | 1b | A | 100% |
| 9 | JAKs inhibitors should be combined with MTX as the first csDMARD choice, although other csDMARDs could be considered in case of MTX intolerance/contraindication | 1b | A | 100% |
| 10 | Combined therapy of MTX with bDMARDs and tsDMARDs does not imply a different management of the standard of care for routine patient safety monitoring | 5 | D | 100% |
Abbreviations: MTX=methotrexate; RA=rheumatoid arthritis; TNF=tumour necrosis factor; ABT=abatacept; RTX=rituximab; mg=milligram; IL-6=interleukin 6; JAKs=Januskinases; bDMARDs=biological disease modifying drugs; tsDMARDs=targeted synthetic disease modifying drugs (DMARDs).
In patients with established RA (but also in early RA) and inadequate response to MTX, different SLR and meta-analyses have shown6,7 that the addition of TNF inhibitors to MTX was superior to the biologics monotherapy in terms of disease activity (ACR20, 50, 70 response), function evaluated with the Health Assessment Questionnaire (HAQ), pain control, patient global assessment or radiographic progression. Although less evaluated (at least in RCTs), the combination of ABT or RTX plus MTX, compared with ABT or RTX monotherapy seems to be more effective as well.8–10
R 2.MTX should not be discontinued in patients with active RA and inadequate response to MTX who start IL-6 inhibitors (LE 1a; GR A; GA 91%)RCTs and observational studies have compared TCZ+MTX combination therapy with TCZ monotherapy in patients with inadequate response to MTX.11,12 Most of them have revealed no statistical differences between groups in outcomes such as pain, function, ACR response or Clinical Disease Activity Index and Simple Disease Activity Index remission rates, especially in the short term. However, in other trials, combined therapy was significantly superior in the ACR/EULAR Boolean remission rate or radiographic progression in the medium/long term.12–14 Similar results have been observed with other IL-6 inhibitors.15
The panel weighed up this heterogeneity and established that, in general, MTX (excluding contraindications) should not be discontinued because there is evidence that supports combination efficacy, especially in the long term. However, there might be cases in which IL-6 inhibitor monotherapy might be a good option (e.g. in case of MTX intolerance), but these cases should be carefully individualized.
R 3. MTX should not be discontinued in patients with active RA and inadequate response to MTX who start JAKs inhibitors (LE 1b; GR A; GA 91%)Although data from RCTs have shown no efficacy differences between tofacitinib (TFC) monotherapy and combination therapy with MTX, a network meta-analysis depicted greater ACR20/50/70 responses with the drugs combination.6 However, due to the study design, these results should be considered cautiously. Moreover, it has been estimated that in absolute terms, 6 (0–12) extra patients out of 100 patients treated with TFC will achieve the ACR50 goal due to concomitant MTX therapy.7
Other SLRs and RCTs have revealed that the ACR response rates are similar when comparing patients on baricitinib (BARI) and MTX combination therapy with BARI monotherapy. However, in patients with no or limited prior DMARD treatment, the change in total Sharp score was significantly higher than MTX monotherapy only when BARI was combined with MTX, not for BARI monotherapy.11,16
As in the case of IL-6 inhibitors, with the evidence collected so far, the panel doesn’t recommend MTX cessation when considering the combination with tsDMARDs, especially with BARI.
R 4. When starting a combined therapy with MTX and bDMARDs in MTX-inadequate responders RA patients, it is recommended to continue with the same MTX doses (LE 1b; GR A; GA 100%)A SLR that addressed MTX doses in biologic trials (most of them in MTX-inadequate responders), as well as other RCTs,17,18 have showed that the maximal MTX doses at the start of the bDMARDs combination were 25mg/week for oral and 15mg/week for parenteral routes, respectively. However, mean MTX doses were frequently around 12.5-15mg/week in these trials.18 In the same way, observational studies have described that maximum MTX doses when combined with bDMARDs are variable and reach up to 25–30mg/week, but mean doses are quite similar to the reported in the RCTs.19 In a randomized controlled trial (MUSICA) analyzing the efficacy of two doses of MTX (7.5mg vs 20mg, orally and/or injectable) in patients with established RA with an insufficient response to MTX (>15mg/week) who started adalimumab, the non-inferiority of the low dose was not met, although some clinical and ultrasound parameters do not reach statistical significance.19
The panel also kept in mind current guidelines in the management of RA.1–4 They recommend maximization of treatment effects that includes reaching an optimal MTX dose within a few weeks and maintaining the maximal dose (25–30mg weekly) for at least 8 weeks if tolerated. The panel supports these recommendations and therefore assumes that in patients with inadequate response to MTX in whom a bDMARD is considered, the maximum tolerated dose should have already been achieved irrespectively of the route of administration. This is the dose and route of administration that should be continued.
R 5. When combining MTX with TNF inhibitors, the dose of MTX should be of at least 10mg/week (LE 1b; GR A; GA 100%)It has been stated that in established RA patients with inadequate response to MTX, MTX at 10mg/week provides additional efficacy to TNF inhibitors (including the reduction of the incidence of antidrug antibodies), while intolerance leading to discontinuation at these low doses was very rare.20 In the randomized trial CONCERTO in MTX naïve RA patients who started adalimumab, efficacy of 10 and 20mg/week MTX appeared equivalent.20 As exposed in the recommendation 4, it is possible that a bDMARD or tsDMARDs is indicated in a patient on MTX below 10mg/week due to toxicity/tolerability. In this cases the previous doses should be continued.
R 6. When starting a combined therapy with MTX and JAKs inhibitors in MTX- inadequate responders RA patients, it is recommended to continue with the same MTX doses (LE 1b; GR A; GA 100%)MTX doses when adding TFC in MTX inadequate responders are different among RCTs, from7.5 to 25mg/week.21,22 In the ORAL Standard trial, patients were receiving weekly 7.5–25mg MTX,23 in the ORAL Sync mean MTX dose was 14mg/week,21 and in the ORAL Scan doses ranged between 15 and 25mg/week.22 Recent real world evidence have shown that in daily practice MTX doses combined with TFC are close to those reported in the RCTs.24
MTX doses in combination with BARI in the published RCTs are also variable,25 mainly from 10 to 25mg/week. Mean doses are quite similar to those in TFC trials, like in the RA-Beam study (15mg/week).25
The same considerations exposed for bDMARDs are applicable for tsDMARDs. A post hoc analysis based on pooled data from 2 RCTs in Japanese patients, showed that tofacitinib efficacy may be unaffected by background MTX dose.26 At month 3, ACR20/50/70 response rates, mean DAS28-4 (ESR) CFB and HAQ-DI CFB were similar across different MTX doses.
R 7. In RA patients who have achieved and sustained the treatment goal, the panel recommends, as treatment strategy, to give priority to the de-escalation of the bDMARDs, which does not exclude MTX dose adjustments or even MTX withdrawal in some intolerant patients on IL-6 inhibitors (LE 5; GR D; GA 100%)The panel agreed that in patients who have achieved and sustained the treatment goal, the de-escalation of the bDMARDs (irrespectively of the type) should be considered. The term ‘sustained’ is still not defined precisely, but at least 6 months was delimited as a minimal time frame. However, on the other hand, this does not exclude MTX dose adjustments (along with the de-escalation of the bDMARDs) or even MTX withdrawal in some intolerant patients on IL-6 inhibitors. Moreover, in some cases, the adjustment of MTX doses could even be the first step (before the de-escalation of the bDMARDs).
In this context, the TNF inhibitor tapering after attainment of remission in early and established RA allows excellent outcomes to be maintained.27–29 The PRESERVE study, a RCT comparing the safety and efficacy of once-weekly ETN 50mg, ETN 25mg, and placebo in combination with MTX, analyzed radiological progression between groups of patients continuing full-dose ETN with patients switching to half-dose or stopping of ETN. There were no differences in the changes of the modified total Sharp score between these groups.27 Recently, in a RCT designed to evaluate the effectiveness of two tapering strategies after achieving controlled disease in patients with RA receiving combination therapy with TNF inhibitors and csDMARDs, for up to 9 months, flare rates when tapering csDMARDs or TNF inhibitors were similar. Moreover, after 1 year, anon-significant difference was found between them.30
On the other hand, in a non-inferiority RCT, RA patients on TCZ+MTX and good/moderate EULAR response were randomized to tapering MTX or continuing stable doses of MTX for 24 weeks.31 Although the study stopped early due to low recruitment, the predetermined non-inferiority criteria were still met. Therefore, tapering MTX in patients with RA receiving TCZ was non-inferior to continuing stable MTX in maintaining a good/moderate EULAR response. In another double-blind RCT in biologic-naïve RA patients with a disease activity score 28 (DAS28)>3.2 despite oral MTX, treatment with TCZ+MTX was prescribed for an initial 16 weeks period. Patients who achieved low disease activity (DAS28 ≤3.2) were randomised to continue with TCZ+MTX or switch to TCZ+placebo for an additional 12 weeks. In both treatment groups, the percentage of patients in clinical remission from 16 to 28 weeks was similar, as were the improvements in disease activity, functional disability and quality of life.32 However, as the authors comment, the short period of 12 weeks on TCZ monotherapy after randomisation in JUST-ACT does not allow for a long term evaluation of response maintenance, which may theoretically influence our results.
The SMART study was an open-label non-inferiority study in patients with an inadequate response to TNF inhibitors in which all patients on RTX 1000mg+MTX and a moderate or good EULAR response were randomized to RTX 1,000mg for 1 or 2 doses.33 Over 104 weeks, the adjusted mean difference in DAS28 area under the curve was 51.4 (95% CI −131.2 to −234), indicating non-inferiority (as it was pre-defined) between the two doses.
Dose reduction of ABA to half-dose (plus other DMARDs mainly MTX) in patients with early RA was evaluated in a sub-study of the AGREE trial.34 At 1 year, 34% (half-dose) and 31% (full-dose) of patients experienced a flare.
Regarding to the JAKs inhibitors, we need more studies to make robust recommendations.
As previously suggested in the Spanish REDOSER project, the panel considered that rheumatologists should individualize every case and consider tapering in clinical situations in which down titration will likely be successful, related primarily to early RA, depth of improvement and duration of remission.35
R 8. bDMARDs should be combined with MTX as the first csDMARD choice, although other csDMARDs could be considered in case of MTX intolerance/contraindication (LE 1b; GR A; GA 100%)TNF inhibitors efficacy and safety in combination with MTX or leflunomide (LEF) was analyzed in the RABBIT biologics registry.36 EULAR response rates after 24 months ranged from 74% to 81% for combinations with MTX and 72% to 81% for LEF (P<.050). The safety profile was the expected when using these drugs. Regarding ABT, a post-hoc exploratory analysis from 3 interventional trials and 1 real-world study, showed the efficacy and safety data extracted from RA studies in which ABT combination with csDMARDs other than MTX was permitted. At 6 months and 2 years, the efficacy (DAS28 and HAQ) was similar for combinations of ABA+MTX and ABA+other csDMARDs like LEF, hydroxychloroquine, SZZ or azathioprine.37 The CERERRA Collaboration (10 European biologics registries) found that significantly more patients achieved a EULAR good response at 6 months when treated with RTX plus mean LEF doses of 20mg/day (29.1%) compared with RTX plus mean MTX doses of 14.4mg/week (21.1%). Similar results were observed at 12 months. Adverse events (AE) occurred in 10.2% and 13.2% of patients, respectively.10 There are also RCTs of IL6 inhibitors that included patients in combined therapy with different csDMARDs but no comparative data were shown.13 A small observational study did not find efficacy or safety differences between TCZ+MTX and TCZ+LEF.38
On the other hand, MTX in combination with TNF inhibitors has largely demonstrated to increase this group of biologics survival.39,40 However, connected to ABT, RTX and IL6 inhibitors, so far MTX has not clearly been associated with longer survival.41,42
Some bDMARDs are associated with immunogenicity (and impact on clinical efficacy and safety) that might be attenuated with the concomitant use of MTX.43–45 The positivity of anti-drug antibodies to TNF inhibitors occurs in about 13% of patients but varies greatly, depending on the specific TNF inhibitors, with the highest rates observed with IFX and ADA, and the lowest with ETN.43–45 Several studies have confirmed that in patients treated with IFX, ADA, CZP or GOL, the combination with MTX decreases the rate of anti-drug antibodies.45,46 Data related to ABT, RTX and IL-6 inhibitors suggest that these drugs are clearly less immunogenic.45,47 However, it should be taken into account that immunogenicity of individual agents has been analyzed using different study designs, treatment duration, RA characteristics, as well as a great immunoassay heterogeneity. Therefore, data interpretation is challenging and should be carefully considered.48
Finally, regarding the use of parenteral MTX, although more data is still needed, observational studies have shown that its efficacy and safety when combined with bDMARDs are similar to those with oral MTX.2,49,50
R 9. JAKs inhibitors should be combined with MTX as the first csDMARD choice, although other csDMARDs could be considered in case of MTX intolerance/contraindication (LE 1b; GR A; GA 100%)Up to 86% of patients from TFC’ RCTs on combined therapy use MTX,51 although there are also some patients taking other DMARDs like LFN, antimalarials, SZZ, sodium aurothiomalate or D-penicillamine.51 However, comparative analyses are not available. BARI RCTs have reported the same data. In terms of survival, a pooled analysis of TFC studies found that up to week 72, the discontinuation rate when receiving combination therapy with a csDMARD (86.2% MTX) was 50.7% vs 45.2% of patients receiving TFC monotherapy.52
Nevertheless, so far TFC and BARI are not immunogenic drugs, and more studies are necessary to assess the role of parenteral MTX in patients with JAK inhibitors.
R 10. Combination therapy of MTX with bDMARDs and tsDMARDs does not imply a different management of the standard of care for routine patient safety monitoring (LE 5; GR D; GA 100%)Pooled data from RCTs have depicted that MTX and bDMARD combination therapy is not significantly associated with an increased risk of serious AE, serious infections or death when compared with bDMARD monotherapy.53 However, an increase risk of gastrointestinal AE(including hepatic AE) has been reported with the combined therapy.7 Observational studies including biologic registries have also found no increased risk in other relevant AE like cancer.54
As a result of all data exposed above, the panel considered that risk management when combining MTX with b- or tsDMARDs should be the same as for their individual components.55
DiscussionIn RA, MTX continues to be the anchor (‘first’) drug both as monotherapy as well as in combination with other drugs.2,56
It has been estimated that, in MTX naïve patients, MTX monotherapy achieves satisfactory disease control in approximate one-third of patients.57 Therefore, an important rate of RA population (with an inadequate response to MTX) will need intensification of therapy which may include addition of a bDMARD or tsDMARD.1–4
We have proposed practical and specific recommendations when using MTX combined therapy with b- or tsDMARDs that provide complementary advice to those from EULAR recommendations and similar documents. These recommendations were based on the best evidence available and achieved high level of agreement among experts.
The first question addressed the need to continue or discontinue MTX in patients with inadequate response to MTX when initiating a bDMARD or tsDMARD. As described in the main text, all bDMARDs have superior efficacy when combined with MTX compared to monotherapy,6–10 even for IL-6 and JAKs inhibitors in many aspects.11,12,14,16 However, the panel also considered that, in reference to RA signs and symptoms or physical function, most of IL-6 and JAKs inhibitors RCTs have shown no differences between biologic monotherapy and combined therapy. This led the experts to definitely recommend the continuation of MTX (if no contraindications are present) with TNF inhibitors, ABT and RTX, and not to discontinue MTX with IL-6 and JAKs inhibitors, in line with EULAR recommendations.1 This proposal is in line with Spanish national and EULAR recommendations1–3 but differ from ACR guidelines that allow the option of bDMARDs monotherapy, even when TNF inhibitors, ABT and RTX are selected.4 For IL-6 and JAK inhibitors, the panel pointed out that in some cases biologic monotherapy could be considered as treatment option.
Next, for patients who start a combination of a csDMARD with b- or tsDMRDs, the panel proposed MTX as first choice. There is plenty of evidence regarding its efficacy, safety profile, and role in biologics immunogenicity and survival.10,37,39–43,45–48 However, the evidence also supports the use of other csDMARDs.13,37,38 Concerning MTX optimal doses, the panel stated that this should be the maximum tolerated as recommended in EULAR and similar consensus documents.1–4 This is a key requirement in daily practice to support the cost-effectiveness of adding a b- or tsCDMARDs, since it has been argued that many RCT let the recruitment of “inadequate” MTX responders with unusual low doses of MTX. In the case of MTX route of administration, when combined with b- or tsDMARDs, oral and parenteral should be considered as previously recommended.2,49,50
Another challenge in daily practice is the treatment strategy in patients that have achieved the treatment goal. In fact, for the panel it is crucial that the target-state should be achieved and sustained. Although the panel fully agreed on prioritizing the b- or tsDMARD de-escalation, especially considering MTX tolerability in RA patients, other scenarios were also accepted. For example, in patients who are not experiencing a proper tolerance to MTX, MTX dose adjustments could be considered along with the de-escalation (even as a first step), or MTX withdrawal in patients on IL-6 and JAK inhibitors.
Finally, safety issues were discussed and analyzed. It could be expected that the combination therapy might increase the frequency of AE. Nevertheless, according to evidence, MTX and b- or tsDMARD combination therapy is not significantly associated with an increased risk of serious AE compared with bDMARD or MTX monotherapy.7,53,54 Therefore, risk management when combining MTX with b- or tsDMARDs should be the already recommended monitorization.1,55 And this is why we just generated one general recommendation.
On the other hand we should comment some limitations of the present project. As described, this was a very ambitious project, with a wide scope. We have tried to answer multiple questions about all available drugs in RA. As a consequence, we decided to perform a SLR based basically on previous SLRs. This means that a single SLR can fall short in obtaining the information necessary to answer all the proposed questions.
In summary, this document provides a series of practical recommendations on the use of MTX in combination with bDMARDs or tsDMARDs. We hope that the current recommendations will find their way into the clinic for a better care of the RA patients in the real-world setting.
FinancingThis Project was financed by Gebro Pharma, Barcelona, Spain.
Conflict of interestJTM has received honoraria from Gebro, Lilly, Pfizer, Roche, Fresenius. The rest of the authors report having no conflicts of interest.