To evaluate the modified dosages of anti-TNF in controlling disease activity in rheumatoid arthritis (RA) measured by DAS28-ESR.
Patients and methodsCross-sectional study: RA patients treated with etanercept (ETN), adalimumab (ADA) or infliximab (IFX), at standard or modified doses. Main variables: dosage, concomitant disease modifying drugs (DMARDs), DAS28-ESR.
Results195 RA patients included (79% women, mean age 58.1 years): ETN=81, ADA=56, IFX=58. Mean disease duration and time to first biological treatment was higher in IFX group (P=.01). Patients distribution by dosage: standard: ETN (72.8%), ADA (69.6%), IFX (27.6%); escalated: IFX (69%), ADA (5.4%), ETN (0%); reduced: ETN (27.1%), ADA (25%), IFX (3.4%). Concomitant DMARDs use was lower in ETN (58.2%) than ADA (66.07%) and IFX (79.31%). Higher proportion of responders (DAS28 ≤3.2) in ADA (65.3%) and ETN (61.7%) than IFX (48.3%).
ConclusionsRA clinical control can be preserved with modified anti-TNF dosages. Controlled prospective studies should be performed to define when therapy can be tailored and for which patients.
Avaluar dosis modificadas de anti-TNF en el control de actividad de la enfermedad en la artritis reumatoide (AR) medida por DAS28-VSG.
Pacientes y métodosEstudio transversal: pacientes con AR tratados con etanercept (ETN), adalimumab (ADA) o infliximab (IFX), en dosis estándar o modificada. Principales variables: dosis, enfermedad concomitante, fármacos modificadores (DMARDs), DAS28-VSG.
Resultados195 pacientes con AR fueron incluidos (79% mujeres, edad media 58,1 años): ETN = 81, ADA = 56, IFX = 58. La duración de la enfermedad y el tiempo medio hasta el primer tratamiento biológico fue mayor en el grupo con IFX (p = 0,01). Distribución de los pacientes por dosis estándar: ETN (72,8%), ADA (69,6%), IFX (27,6%); incremento: IFX (69%), ADA (5,4%), ETN (0%), reducción: ETN (27,1%), ADA (25%), IFX (3,4%). Uso concomitante DMARD fue menor en ETN (58,2%) que ADA (66,07%) e IFX (79,31%). La mayor proporción de respondedores (DAS28 ≤ 3,2) se vio en ADA (65,3%) y ETN (61,7%) que en IFX (48,3%).
ConclusionesEl control clínico de la AR se puede preservar con dosis modificadas de anti-TNF. Estudios prospectivos controlados deben realizarse para definir cuando la terapia se puede adaptar y en que pacientes.
Rheumatoid arthritis (RA) is a chronic, systemic, inflammatory disease with a fluctuant but progressive course, leading, without treatment, to cartilage damage, bone erosions, and joint destruction, the main causes of long-term disability.1
Although the registered doses of anti-TNFs are at the top of the group dose response curve and under-treatment is not expected to be very frequent, the use of standard dosages in clinical practice may result in under or over treatment2 once remission or low disease activity have been achieved, making it reasonable to attempt treating patients on an individualized schedule.
Clinical experience confirms the long-term efficacy of standard dosages for anti-TNF drugs, evaluation is still necessary to determine if reduced dosages could maintain clinically controlled patients. There are some reports addressing the issue of stopping, lowering, or increasing the dosages inpatients in remission,3,4 showing that up to 50% of patients relapse if the drug is stopped, and reintroduction of the therapy might not achieve previous results. However, tapering anti-TNF dosages seems to preserve the clinical efficacy in most patients.5 Consequently, RA patients treated with anti-TNF who are in maintained remission6 could benefit from treatment adjustment in order to find the lowest effective dose.
The aim of the study was to assess the anti-TNF dosages in RA patients, evaluating whether modified patterns could control patients clinically under our daily clinical practice.
Patients and MethodsStudy Design and Patient PopulationA cross-sectional, non-interventional study was conducted in the Hospital General Universitario Gregorio Marañón (HGUGM) in Madrid (Spain), from October 2010 to October 2011. During the study period, patients receiving anti-TNF for a minimum period of 12 months, and attending per routine follow-up the Biological Therapy Unit at the Rheumatology Department, were subsequently included. Requirements included being diagnosed with RA, according to the American College of Rheumatology's (ACR) 1987 revised criteria,7 and treated with any of the following anti-TNF drugs: etanercept (ETN), adalimumab (ADA), or infliximab (IFX),administered either at standard or modified doses. Standard therapy was that in accordance with the approved prescribing information: ETN 25mg twice a week or 50mg weekly, ADA40mg every other week, and IFX 3mg/kg every 8 weeks. Modified therapy was either escalated (the time between doses was shorter or the doses were higher than the standard ones) or reduced (the time between doses was longer or the doses were lower than the standard ones). Dose tapering was adopted if the patient was in remission by DAS28 <2.6 or low activity <3.2 for those with very established and chronic disease for at least 1 year, based on a clinician and patient agreement, and not following any standardized protocol.
Study VariablesThe variables studied were the percentage of patients treated with each drug (IFX, ADA, and ETN), the prescribed dosages, the distribution of patients in clinical categories according to the Disease Activity Score (DAS28):11 Remission (DAS28 <2.6); Low activity (DAS28: 2.6–3.2); Moderate activity (DAS28: 3.2–5.1); or High activity (DAS28 >5.1) in each treatment group, and the concomitant treatment with DMARDs: methotrexate (MTX), leflunomide (LF), sulfasalazine, andhydroxychloroquine.
EthicsThe study was approved by the HGUGM Ethics Committee and all participants provided written informed consent.
Statistical AnalysesData was analyzed with the SPSS 17.0 statistical program. Continuous variables were described using mean and standard deviation (SD) or median and range. Categorical variables were described with frequencies and percentages. Statistical analyses including parametric and non-parametric tests in relation with variables distribution were performed comparing the 3 groups (ANOVA or Kruskal–Wallis test). Additional comparisons between IFX-ADA, IFX-ETN, and ETN-ADA groups were carried out using non-parametric or parametric tests (U Mann–Whitney, Chi square).
ResultsDemographic and Clinical Data (Table 1)195 RA patients were included. Participants were predominantly women (79%) and the global mean age was 58.1 (±14.9) years. Patients were distributed into 3 groups, according to the anti-TNF drug used: 81 patients in the ETN group (41.5%), 56 in the ADA group (28.7%), and 58 in the IFX group (29.7%). Mean disease duration (years) was statistically different between groups (P=.01) although most patients (42.2%) had a mean disease duration between 10 and 20 years. In the 2–5 year range of duration, however, the percentage of patients in the ADA group was significantly higher when compared to ETN and IFX (P=.03). There were also significant differences between groups in terms of time from diagnosis to the administration of the first biological treatment. IFX was most commonly used as first-line biological treatment, whereas ETN was the option most commonly used as second-line.
Demographic and clinical characteristics of patients.
| ETN (n: 81) | ADA (n: 56) | IFX (n: 58) | P value | |
| Demographics | ||||
| Female; n (%) | 64 (80) | 38 (67.9) | 52 (89.7) | ns |
| Age (years; mean±SD) | 53.2 (±15.3) | 59.9 (±15.7) | 61.3 (±10.5) | ns |
| DAS28 (mean±SD) | 2.75 (±1.5) | 2.69 (±1.5) | 3.25 (±1.5) | ns |
| Concomitant DMARD | 58.0% | 66.1% | 79.3% | P=.01 |
| Clinical disease duration (years; mean±SD) | 11.3 (±6.3) | 10.5 (±6.5) | 13.2 (±6.0) | P=.01 |
| <2 years | 2.7% | 0% | 0% | ns |
| 2–5 years | 6.8% | 21.6% | 10.9% | P=.03 |
| 5–10 years | 36.5% | 31.4% | 18.2% | ns |
| 10–20 years | 41.9% | 37.3% | 47.3% | ns |
| >20 years | 12.2% | 9.8% | 23.6% | ns |
| Time from diagnosis to first anti-TNF treatment (years; mean±SD) | 5.7 (±5.6) | 5.9 (±5.6) | 7.5 (±4.6) | P=.01 |
| <2 years | 17.6%* | 11.8%* | 5.5% | P=.02* |
| 2–5 years | 25.7% | 31.4%* | 23.6% | P=.02* |
| 5–10 years | 27.0% | 31.4% | 29.1% | ns |
| 10–20 years | 18.9% | 23.5% | 40.0% | ns |
| >20 years | 4.1% | 2.0% | 0% | ns |
| First-line biological option (%) | 63.2 | 70.9 | 87.3 | P=.02 |
| Second-line biological option (%) | 30.9 | 16.7 | 7.3 | P=.02 |
NS: no significant. ETN: etanercept; ADA: adalimumab; IFX: infliximab; DAS28: Disease Activity Score.
P value: ANOVA test between the 3 groups.
Most patients in ETN (72.8%) and ADA (69.6%) groups were treated with the standard dosages. In the ETN group there were no patients in escalated doses while most patients in IFX had an escalated regimen (69%). The percentage of patients with reduced doses were 27.1% and 25% for both the ETN and ADA groups respectively, with less than 4% in the IFX group.
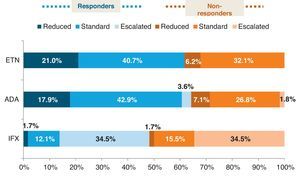
The distribution of patients in the ETN and ADA groups were similar, with most patients receiving the standard therapy, whereas in the IFX group there was a considerable proportion of patients with an escalated regimen. We found more than 60% of responders (DAS28 ≤3.2) in the ETN and ADA groups and less than 50% in the IFX one. Fig. 1 shows the distribution of responder and non-responder patients within the different treatment regimens (standard, escalated, and reduced) of the evaluated drugs. Both ETN (61.7%) and ADA (64.3%) groups had more responders than non-responders in any of the dosage regimens, even in the reduced one. On the other hand, the IFX group, despite having the higher number of patients in an escalated regimen, had globally less responders (48.3%), even at increased doses, where responders and non-responders were distributed at 50%. However, mean DAS28 value was similar for all anti-TNF (ETN: 2.8±1.5, ADA: 2.7±1.5,IFX: 3.3±1.5; P=.6). ETN patients, regardless of biologic line, were less likely to use concomitant DMARD than ADA or IFX patients. The drug more frequently used in association was methotrexate (MTX) followed by leflunomide (LEF).
Percentage of responders (DAS28 ≤3.2) and non-responders (DAS28 >3.2) in the different dosage regimens of anti-TNF treatments. ETN: etanercept; ADA; adalimumab; IFX: infliximab. Reduced (the time between doses was longer or the doses were lower than the standard ones). Standard (according to the approved prescribing information): ETN 25mg twice a week or 50mg weekly, ADA 40mg every other week, and IFX 3mg/kg every 8 weeks. Escalated (the time between doses was shorter or the doses were higher than the standard ones).
Although clinical experience confirms the long-term efficacy of standard dosages for anti-TNF drugs, this study, based on our daily clinical experience, provides real-life data on the use of different dosage regimens. While the mean DAS28 value was similar between groups, we found more responders by DAS28 among patients in ETN and ADA groups when compared to IFX group, despite the higher percentage of patients with dose escalation in the latter group. IFX dose escalation in clinical practice has been reported elsewhere.8–15 Nevertheless, in our cohort IFX was mainly used as a first line therapy, having a longer disease duration as well as a longer delay until biological treatment. The election of the drug and the different efficacies between anti-TNFs might be related to the patient's clinical and demographic characteristics, as well as the drugs available at the moment of starting biological treatment as well as the recent tendency to treat to target.16
Nevertheless, when patients achieve clinical response, physicians can be constrained by the dosages specified in the summary of product characteristics (SPC). Real-life clinical practice supports the benefits of tailoring RA treatment, considering the severity of the condition, the effectiveness of each specific drug, and the risk of side effects. In our study over 40% of the patients were treated with either higher or lower dosages than those recommended in the approved prescribing information.
Considering the cross-sectional design of the study, the interpretation of the results is limited since we cannot differentiate cause and effect from a simple association. In this sense, we have no information regarding patients in which dose reduction had been previously attempted without maintaining clinical response, and those who never tried dose titration. Also, we have identified some patients with very active disease or moderate disease activity on reduced dosage regimens. Even though this finding might be contradictory, those patients presented very established RA with chronic damage due to long-term disease duration, but no current clinical activity.
DMARD use was lower for patients on ETN when compared to ADA and IFX. Better clinical efficacy of anti-TNF in combination with DMARD is broadly accepted,17 however it is interesting to highlight the increased possibility of clinical control in our ETN group without concomitant DMARD, in accordance with other reports which give data of up to 50% of patients on ETN treatment inmonotherapy.18 However, non use of DMARDs have been broadly showed in patients after various biologics, and taking into account that ETN was the second line for most of the patients included n this study we cannot firmly conclude monotherapy as a good option only for ETN.
Despite its limitations, this study has some strengths, since cross-sectional studies are useful at identifying associations and generating hypotheses.19 This study may be considered a good start point for controlled long-term trials that further investigate our findings. When a drug is effective at achieving disease control, it is important to consider the possibility of tapering doses whenever possible to reduce the cost and the possible long-term side effects.
ConclusionsRegarding our results, it seems reasonable, when clinical response has been achieved, to try to reduce the standard dosage in order to establish the minimum effective dose, because tapered doses do not seem to lead to high disease activity in some patients.
Conflict of InterestThe authors declare no conflict of interest.
Ethical ResponsibilitiesProtection of Human and Animal SubjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of DataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to Privacy and Informed ConsentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.