To investigate if patients with Systemic Sclerosis (SSc) show a higher prevalence of neuropathic pain (NP) in comparison with controls. To study the relationship between clinical variables of the disease and NP among SSc patients.
Material and methods48 patients and 45 controls were included. Presence of NP was assessed applying the DN4 “Douleur Neuropathique en 4 Questions” questionnaire. Different clinical variables were also assessed in patients. Statistical analysis included parametric, nonparametric tests and multivariate logistic regression.
ResultsNP was significantly higher in SSc patients (56.2% vs 13.3%, p<0.001). Mean Modified Rodnan Skin Score was independently associated with the presence of NP (p<0.05, OR 1.90).
ConclusionsPeripheral nervous system involvement in SSc is not well studied and, as far as the authors are aware, this is the first study published evaluating NP in SSc patients and controls. These findings should raise the awareness of the clinician to recognize and address the presence of NP in these patients, especially in those with severe skin involvement.
Valorar si los pacientes con esclerosis sistémica (ES) presentan una mayor prevalencia de dolor neuropático (DN) respecto a un grupo control. Estudiar la asociación entre variables clínicas y DN entre los pacientes.
Material y métodosFueron evaluados 48 pacientes con ES y un grupo control de 45 individuos. La presencia de DN fue determinada utilizando el cuestionario DN4 «Douleur Neuropathique en 4 Questions». Diferentes variables clínicas se evaluaron entre los pacientes. El análisis estadístico incluyó test paramétricos, no paramétricos y regresión logística multivariable.
ResultadosLa presencia de DN fue significativamente mayor en el grupo de pacientes (56,2 vs. 13,3%; p<0,001). La media de Modified Rodnan Skin Score se asoció de manera independiente a la presencia de DN (p<0,05; OR: 1,90).
ConclusionesLa afectación del sistema nervioso periférico en la ES no está bien estudiada. Según el conocimiento de los autores, este es el primer estudio publicado en evaluar el DN en estos pacientes respecto a un grupo control. Estos hallazgos deberían llamar la atención para reconocer la presencia de DN en estos pacientes, especialmente si existe afectación cutánea grave.
Rheumatic diseases are frequently chronic multi-systemic conditions and pain is a common debilitating symptom. Different types of pain including nociceptive and neuropathic are well recognized in diseases such as systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA).1
Systemic Sclerosis (SSc) is a rheumatic disorder characterized by fibrosis of skin and internal organs, vasculopathy and systemic inflammation.2 To date, few studies assessed pain in SSc patients and conclusions are not clear.3–6 Specific measurements for pain evaluation in these patients are lacking as well as specific treatment recommendations. However, pain is often one of the most incapacitating complaints of SSc patients being responsible for decreased quality of life and well-being.4–7 Besides the well-known nociceptive component, neuropathic pain (NP) may also play an important role in the clinical picture of the disease. Patients with SSc frequently complain of distal paraesthesia and pain affecting the extremities However, due to the absence of data regarding this condition, the burden related to NP in SSc patients is not actually known.
Evaluating the neuropathic component of pain in SSc patients may allow not only estimating the real prevalence and burden from this condition but also defining strategies to diagnose and treat symptoms of this disorder. Moreover, investigating the relationship between the presence of NP and specific clinical features of the disease may help to identify patients at higher risk of NP and its complications.
Therefore, the objective of this study was to determine if patients with SSc have a higher prevalence of NP compared with a group of age and sex matched controls and study possible associations between NP and SSc clinical variables.
Material and methodsA cross-sectional study was conducted including 48 patients diagnosed with SSc, fulfilling SSc classification criteria of the American College of Rheumatology (ACR)8 and followed up at a Portuguese Rheumatology Department and a group of 45 matched control subjects. Consecutive SSc inpatients were included between September and December 2016. The control group comprised healthy subjects randomly selected from the Hospital working staff, based on age, gender and race distribution of the patients group; this recruitment process took place during the same period of time.
Exclusion criteria included age <18 years, diagnosis of other chronic inflammatory rheumatic disease or known neurological disorder. Subjects chronically medicated with drugs commonly used to treat NP with proven efficacy (including neuroleptics, tricyclic antidepressants and local anaesthetics) were also excluded (defined as daily intake for at least 4 weeks before study evaluation). Individuals diagnosed with the following medical conditions with possible association with NP – Diabetes Mellitus, thyroid disease, history of carpal tunnel syndrome surgery, were included as the prevalence of these pathologies was similar in the patients and control group (Table 1).
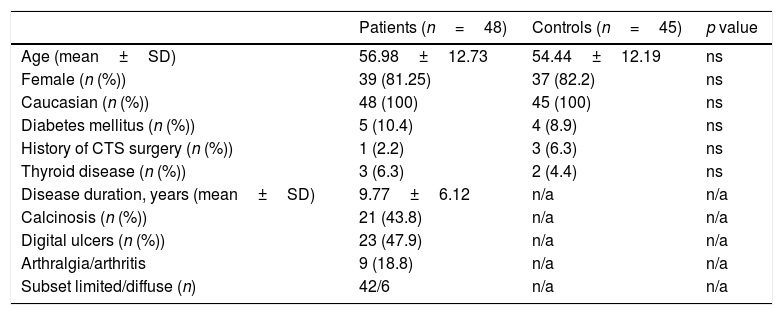
Baseline characteristics of patients with Systemic Sclerosis and controls groups.
| Patients (n=48) | Controls (n=45) | p value | |
|---|---|---|---|
| Age (mean±SD) | 56.98±12.73 | 54.44±12.19 | ns |
| Female (n (%)) | 39 (81.25) | 37 (82.2) | ns |
| Caucasian (n (%)) | 48 (100) | 45 (100) | ns |
| Diabetes mellitus (n (%)) | 5 (10.4) | 4 (8.9) | ns |
| History of CTS surgery (n (%)) | 1 (2.2) | 3 (6.3) | ns |
| Thyroid disease (n (%)) | 3 (6.3) | 2 (4.4) | ns |
| Disease duration, years (mean±SD) | 9.77±6.12 | n/a | n/a |
| Calcinosis (n (%)) | 21 (43.8) | n/a | n/a |
| Digital ulcers (n (%)) | 23 (47.9) | n/a | n/a |
| Arthralgia/arthritis | 9 (18.8) | n/a | n/a |
| Subset limited/diffuse (n) | 42/6 | n/a | n/a |
Abbreviations. ns: not significant; n/a: not applicable; SD: standard deviation; CTS: carpal tunnel syndrome.
Patients and controls were consecutively evaluated and the Portuguese translated version of the DN4 questionnaire was used for assessing the presence of NP.9 DN4 “Douleur Neuropathique en 4 Questions” is a simple and objective screening tool that allows the distinction between NP and nociceptive pain. It comprises 4 groups of questions consisting of 7 sensory descriptors and 3 signs related to a sensorial exam. According to the total final score obtained, subjects were classified as NP positive if a score of 4 or higher was present.9,10 The same observer was responsible for evaluating all subjects in order to reduce subjectivity inherent to physical examination.
Additionally, in SSc patients, skin involvement was also evaluated clinically by the Modified Rodnan Skin Thickness Score (mRSS) ranging from 0 to 51. Hand mobility (HAMIS) and SSc Severity Scale (SScSS) were also calculated and relevant clinical variables of the disease were collected.
Parametric and nonparametric tests were used for comparative analysis. Multivariate logistic regression was used to identify factors associated to NP. Statistical significance was defined as p<0.05.
The study was performed following the Declaration of Helsinki principles and informed consent was obtained from all subjects.
ResultsDemographic and basal clinical data of patients and controls is summarized in Table 1.
Regarding the defined clinical scores calculated in the patients group, we obtained the following results:
Total mRSS ranged from 2 to 43 with a median of 15 (7–22.75) (interquartile range [IQR]), HAMIS ranged from 0 to 24 with a median of 4.5 (1–10 [IQR]) and SScSS ranged from 2 to 22 with a median of 7.0 (4–9 [IQR]).
In this study, prevalence of NP assessed by DN4 questionnaire was significantly higher in SSc patients comparative to controls (56.2% versus 13.3%, p<0.001).
In SSc patients, NP presented with “numbness” (83.3%), “tingling” (54.2%), “pins and needles” (47.9%), “painful cold” (47.9%), “itching” (25%), “electric shocks sensation” (22.9%) and “burning” (4.17%) mainly in extremities. In the control group, the percentages were much lower; the most prevalent symptom was “tingling” (24.4%). Regarding physical examination, hypoesthesia to touch was present in 45.83% of patients (versus 11.1% of controls) and hypoesthesia to pinprick in 37.5% (versus 8.9% of controls).
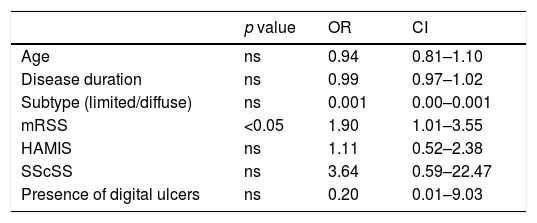
Among SSc patients, applying univariate analysis tests, the authors found that the presence of arthralgia or arthritis (all non-erosive) and the presence of calcinosis were not associated with NP (p=0.124 and p=0.062, respectively). On the other hand, NP was more prevalent in older patients (p=0.032), in diffuse subtype (p=0.024), in longer disease duration (p=0.04), in the presence of digital ulcers (DU) (p=0.018) and in patients with higher mRSS (p<0.001), higher HAMIS (p<0.001) and higher SScSS (p<0.001). Subsequently, a multivariate logistic regression analysis was performed regarding the association of previous variables and NP and it was found that only mean mRSS was independently associated with the presence of NP (Table 2).
Association between NP and clinical variables among patients with Systemic Sclerosis in a multivariate logistic regression analysis.
| p value | OR | CI | |
|---|---|---|---|
| Age | ns | 0.94 | 0.81–1.10 |
| Disease duration | ns | 0.99 | 0.97–1.02 |
| Subtype (limited/diffuse) | ns | 0.001 | 0.00–0.001 |
| mRSS | <0.05 | 1.90 | 1.01–3.55 |
| HAMIS | ns | 1.11 | 0.52–2.38 |
| SScSS | ns | 3.64 | 0.59–22.47 |
| Presence of digital ulcers | ns | 0.20 | 0.01–9.03 |
Abbreviations. ns: not significant; OR: odds ratio; CI: 95% confidence interval.
NP is a complex symptom known to be caused by lesions to the somatosensory nervous system that alter its structure and function; as a consequence, pain occurs spontaneously and responses to noxious and innocuous stimuli are pathologically amplified. NP can be divided into peripheral or central pain based on the neural lesion location. Multiple and complex mechanisms are responsible for NP, including changes in gene expression both at peripheral and central NP: ectopic activity due to changes in ion channels lead to peripheral NP and synaptic facilitation with loss of inhibition at different levels of neuroaxis produce central amplification in central NP.11
Regarding SSc patients, the physiopathology of the disease and the basis for neurological damage in this population are not clear. Nervous system involvement, either central or peripheral, is not well recognized and studied. Few previous studies addressed this issue and conclusions were not linear.3,12–14 In 2013, Amaral et al.12 performed a systematic review of data published on neurologic involvement in Scleroderma. They found a prevalence of headache, seizures and cognitive impairment in SSc patients of 23.73%, 13.56% and 8.47%, respectively. Also, depression (73.15%) and anxiety (23.95%) were frequent. Concerning peripheral nervous system involvement, myopathy (51.8%), trigeminal neuropathy (16.52%), peripheral sensorimotor polyneuropathy (14.25%) and carpal tunnel syndrome (6.56%) were the most frequent conditions identified. Autonomic neuropathy related to cardiovascular and gastrointestinal syndromes has also been described. None of these studies aimed to evaluate the prevalence of NP.3,12–14 Another study conducted by Perrot et al. compared the prevalence of different domains of pain between SSc and RA patients. Pain frequency was similar in the two groups, including the presence of a neuropatic component. However, SSc patients had lower pain intensity and dimension scores compared with RA; nevertheless, patients with diffuse cutaneous subtype had higher pain scores than limited subset.15
Thus, to the best of our knowledge, this is the first study evaluating the prevalence of NP in SSc patients in comparison with age, race and sex matched controls. In this cohort, symptoms suggesting sensitive NP component were common in patients with SSc with more than half of them (56.2%) scoring positive for NP according to the DN4 questionnaire. This percentage was significantly higher compared with the matched control group. Additionally, according to this study, different clinical variables of the disease seem to be associated to the SSc patient's individual risk for NP. Interestingly, skin thickness assessed by mRSS was identified as an independent factor associated to NP in these patients. Patients with higher skin thickness assessed by mRSS showed higher NP scores. These findings raise the pertinent question if specific components of skin and subcutaneous tissue may be altered in patients with thicker skin and therefore related to NP mechanisms.
Although these results seem exciting, this study has some limitations, namely, the relatively small sample size and the control group selection process (subjects included were all hospital working professionals which may represent a selection bias and there may be also a confounding by the matching factors). Also, the DN4 questionnaire estimated sensitivity and specificity to correctly identify patients with NP is limited to 86 and 89%, respectively.10 Due to these limitations, the authors highlight the need for more studies in this field to draw precise conclusions. Nevertheless, the authors emphasize the clinician's role of being aware of and recognize the presence of NP in SSc patients, especially in those with severe skin involvement.
Conflict of interestThe authors declare that they have no conflicts of interest.