To evaluate the association between the clinical activity of RA patients and serum adipocytokines (Leptin, Adiponectin and Resistin) and inflammatory cytokines.
MethodsAll RA patients fulfilled ACR 1987 criteria and were treated with DMARDs. Adipocytokine and inflammatory cytokine levels were evaluated using ELISA.
Results121 patients were included in the study. Stratifying according to DAS28 (low, moderate and high activity), there were significant differences for Leptin, Resistin, IL-6 and IL-17, however, no differences were seen for Adiponectin, TNFα or IL-1β. Clinical activity positively correlated with Leptin, Resistin, IL-17 and IL-6 levels, but not with Adiponectin, TNFα or IL-1β. Adiponectin levels negatively correlated with TNFα and positively correlated with IL-1β. IL-1β positively correlated with IL-6 and negatively correlated with TNFα and IL-17.
ConclusionCirculating Leptin, Resistin, IL-6 and IL-17 levels positively correlate with RA clinical activity in a manner independent of the subject's BMI. Complex relationships between inflammatory cytokines were observed in RA patients suggesting that other metabolic or inflammatory factors could be involved.
Evaluar la asociación entre la actividad clínica de pacientes con Artritis reumatoide y adipocitocinas séricas (Leptina, Adiponectina y Resistina), citocinas inflamatorias (TNFα, IL-1β, IL-6, IFNγ e IL-17A).
MétodosSe seleccionaron pacientes con AR (ACR 1987) tratados con FARMEs. Los niveles de adipocitocinas y citocinas inflamatorias fueron evaluados por ELISA.
Resultados121 pacientes se incluyeron en el estudio. La actividad clínica correlacionó positivamente con Leptina, Resistina, IL-6 e IL-17 pero no para Adiponectina, TNFα o IL-1β. Los niveles de Adiponectina se asociaron negativamente con TNFα y positivamente con IL-1β. Por su parte, IL-1β se asoció de manera positiva con IL-6 y negativamente con TNFα e IL-17.
ConclusiónLos niveles circulantes de Leptina, Resistina, IL-6 e IL-17 se asociaron de manera positiva con la actividad clínica de pacientes con AR, independientemente del índice de masa corporal (IMC). Asimismo, en los pacientes con AR se observaron asociaciones complejas entre las adipocitocinas y citocinas, sugiriendo que otros factores tanto metabólicos como inflamatorios pudieran estar involucrados.
In recent years there has been a lot of interest in studying the relationship between adipocytokines and disease activity in Rheumatoid Arthritis (RA), however results are heterogeneous. Several studies have observed higher peripheral blood concentrations of Leptin in RA than in healthy subjects,1 as well as an association with some inflammatory markers2 and disease activity.3 Additionally, other studies have observed an association with insulin-resistance4 and endothelial activation5 but not with radiographic progression.6 In a two-year-follow up study of patients with RA, a positive correlation between Leptin and IL-17 and a decrease in DAS28 was seen in patients treated with DMARDs.2 Similarly, high levels of Adiponectin have also been reported in RA1 and associated with radiographic progression.6
Resistin has been also studied in RA, with heterogeneous findings; some studies reported an association with inflammatory markers7 and clinical activity.8 Baseline Leptin levels predict clinical activity and response to DMARD treatment in non-overweigh/non-obese RA patients,9 suggesting both a role for Leptin in the systemic abnormalities seen during the course of the disease, as well as the influence of adiposity on the RA inflammatory process. However, the association between adipocytokines with inflammatory cytokine levels is poorly understood. Therefore, the objective of this study is to describe the relationship between Leptin, Adiponectin and Resistin with the disease activity of RA patients and the levels of several inflammatory cytokines.
Material and methodsPatient selectionPatients were recruited at the Rheumatology clinic of the Hospital General de Cuernavaca in Morelos, Mexico between 2007 and 2009. The study protocol was reviewed and approved by the Hospital ethics’ committee. All patients followed a similar treatment strategy: methotrexate (7.5–15mg/week) in combination with cloroquine 150mg/d and prednisone ≤10mg/day. Inclusion criteria: Patients included were seen consecutively and had Rheumatoid Arthritis, classified according to the American College of Rheumatology 1987 criteria10; their ages ranged from 18 to 75 years; signed informed consent was obtained from all of the patients and they all had a complete clinical file at the hospital. Disease activity was measured using DAS28 based on the number of swollen and tender peripheral joints, the patients’ overall assessment by visual analog scale (VAS) and the erythrocyte sedimentation rate (ESR). The patients underwent a clinical evaluation performed by a single rheumatologist; venous blood samples were withdrawn afterwards and plasma samples were separated on the same day of collection and frozen at −75°C. All patients received combination treatment with DMARDs (mainly methotrexate and cloroquine) and, in some cases, 10mg or less per day of prednisone. NSAID and analgesics were prescribed on demand. No patients were treated with biologics due to health coverage limitations. For the comparative analysis, patients were stratified according to DAS28 into the following groups: low disease activity (DAS28 ≤3.5), moderate activity (DAS28 >3.5 to ≤5.1) and high disease activity (DAS28 >5.1).
Quantification of adipocytokine and cytokine blood levelsLeptin, Adiponectin, Resistin, IL-1β, IL-6, IL-17 and TNFα levels were determined from venous blood plasma by optimized ELISA employing specific monoclonal antibodies (Santa Cruz Biotechnology, eBioscience). Calibrated curves were prepared in each plate employing a recombinant human standard for each metabolite (Peprotech Inc.). All essays were performed by in triplicate for each sample. Calibration curves fitted a linear regression with a correlation higher than 0.85 and a p value <0.05%.
Statistical analysisDescriptive statistics were employed and results were expressed as means and standard deviations. A one way ANOVA (Kruskal–Wallis test) was performed to compare DAS28-patients subgroups. The comparison between groups was done using the Mann–Whitney test and correlation between variables was determined using Spearman's test (PRISM v.6.0), controlling for gender, age, years since onset of disease, BMI and ESR (STATA v.13). The level of statistical significance was set at 0.05%.
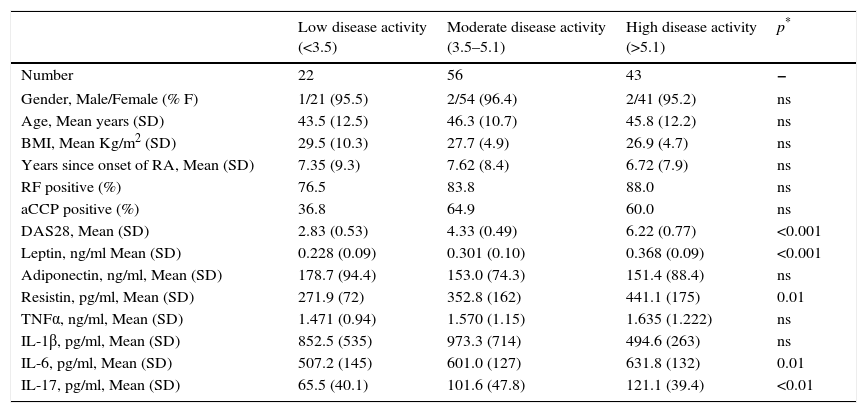
Results121 patients with RA were included in the study. It is worth recognizing there were differences in the number of patients for each activity subgroup, with only 22 patient in the low activity group, while 56 patients had moderate activity (Table 1). However, the three subgroups were similar in gender, age, BMI, time since onset of disease and RF or anti-CCP antibody titers. Although most patients were overweight, no differences were seen between the activity subgroups.
General and immunological data of the total of RA patients and according to their clinical activity subgroup.
| Low disease activity (<3.5) | Moderate disease activity (3.5–5.1) | High disease activity (>5.1) | p* | |
|---|---|---|---|---|
| Number | 22 | 56 | 43 | − |
| Gender, Male/Female (% F) | 1/21 (95.5) | 2/54 (96.4) | 2/41 (95.2) | ns |
| Age, Mean years (SD) | 43.5 (12.5) | 46.3 (10.7) | 45.8 (12.2) | ns |
| BMI, Mean Kg/m2 (SD) | 29.5 (10.3) | 27.7 (4.9) | 26.9 (4.7) | ns |
| Years since onset of RA, Mean (SD) | 7.35 (9.3) | 7.62 (8.4) | 6.72 (7.9) | ns |
| RF positive (%) | 76.5 | 83.8 | 88.0 | ns |
| aCCP positive (%) | 36.8 | 64.9 | 60.0 | ns |
| DAS28, Mean (SD) | 2.83 (0.53) | 4.33 (0.49) | 6.22 (0.77) | <0.001 |
| Leptin, ng/ml Mean (SD) | 0.228 (0.09) | 0.301 (0.10) | 0.368 (0.09) | <0.001 |
| Adiponectin, ng/ml, Mean (SD) | 178.7 (94.4) | 153.0 (74.3) | 151.4 (88.4) | ns |
| Resistin, pg/ml, Mean (SD) | 271.9 (72) | 352.8 (162) | 441.1 (175) | 0.01 |
| TNFα, ng/ml, Mean (SD) | 1.471 (0.94) | 1.570 (1.15) | 1.635 (1.222) | ns |
| IL-1β, pg/ml, Mean (SD) | 852.5 (535) | 973.3 (714) | 494.6 (263) | ns |
| IL-6, pg/ml, Mean (SD) | 507.2 (145) | 601.0 (127) | 631.8 (132) | 0.01 |
| IL-17, pg/ml, Mean (SD) | 65.5 (40.1) | 101.6 (47.8) | 121.1 (39.4) | <0.01 |
There was a significant difference in Leptin, Resistin, IL-6 and IL-17 levels when comparing between activity subgroups using one-way ANOVA (Table 1). In the particular case of Leptin, when comparing DAS28 subgroups, all showed mutual statistical differences (p<0.001), suggesting a positive relation with the increase in the DAS28 score. In a similar manner, a significant mean difference for Resistin levels was observed by comparing the low vs. high activity subgroups (p<0.0007); however, this was not the case when comparing the moderate vs. high activity subgroups (p=0.080) or when comparing the low vs. moderate activity subgroups (p=0.107). In contrast, Adiponectin did not shown differences between clinical activity subgroups. For its part, the low activity subgroup showed lower IL-6 levels than the moderate (p=0.015) or high activity subgroups (p=0.004). Similarly, the low activity subgroup also showed lower IL-17 levels compared to the moderate (p=0.016) or high activity (p=0.0003) subgroups.
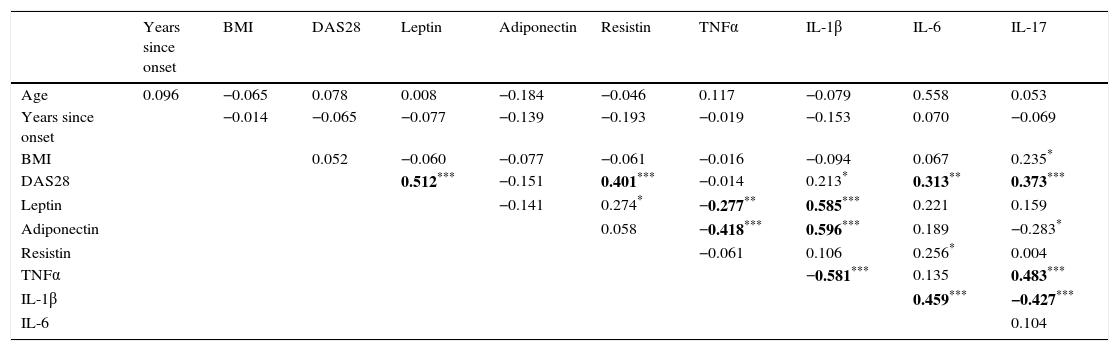
Leptin levels positively correlated with DAS28 (rho=0.513, p<0.0001, Table 2) but not with age, the time since onset of disease or BMI. There was also an important correlation between Leptin and IL-1β (rho=0.585, p<0.0001), suggesting a relationship between this adipocytokine and activation of the immune response. Significant correlations were also observed with Resistin (rho=0.274, p<0.05) and TNFα (rho=−0.277, p<0.005). Likewise, Resistin levels showed a positive relationship with DAS28 (rho=0.403, p<0.0001) but not with the other clinical parameters. Additionally, Resistin only showed a positive significant association with Leptin (rho=0.277, p<0.001) and IL-6 levels (rho=0.256, p<0.05). For its part, the same analysis confirmed the absence of an association of Adiponectin with disease activity (rho=−0.151, p>0.05, Table 2), however it showed a negative correlation with TNFα (rho=−0.418) and IL-17 (−0.283, p<0.05), and a positive correlation with IL-1β (rho=0.596).
Correlation between circulating adipocytokines and inflammatory cytokines.
| Years since onset | BMI | DAS28 | Leptin | Adiponectin | Resistin | TNFα | IL-1β | IL-6 | IL-17 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.096 | −0.065 | 0.078 | 0.008 | −0.184 | −0.046 | 0.117 | −0.079 | 0.558 | 0.053 |
| Years since onset | −0.014 | −0.065 | −0.077 | −0.139 | −0.193 | −0.019 | −0.153 | 0.070 | −0.069 | |
| BMI | 0.052 | −0.060 | −0.077 | −0.061 | −0.016 | −0.094 | 0.067 | 0.235* | ||
| DAS28 | 0.512*** | −0.151 | 0.401*** | −0.014 | 0.213* | 0.313** | 0.373*** | |||
| Leptin | −0.141 | 0.274* | −0.277** | 0.585*** | 0.221 | 0.159 | ||||
| Adiponectin | 0.058 | −0.418*** | 0.596*** | 0.189 | −0.283* | |||||
| Resistin | −0.061 | 0.106 | 0.256* | 0.004 | ||||||
| TNFα | −0.581*** | 0.135 | 0.483*** | |||||||
| IL-1β | 0.459*** | −0.427*** | ||||||||
| IL-6 | 0.104 |
Spearman correlation coefficient, *p<0.05; **p<0.01;***p<0.001.
Bold means P ≤ 0.01.
Both IL-6 and IL-17 showed a significant positive correlation with DAS28 (rho=0.313, p<0.005; rho=0.373, p<0.0001 for IL-6 and IL-17, respectively). On the other hand, TNFα levels were not associated with clinical activity (Table 2). For its part, a significant correlation between DAS28 and IL-1β (rho=0.213, p<0.05) was seen, although it was not possible to differentiate by activity subgroups (Table 1).
DiscussionThe present study supports previous ones suggesting that Leptin and Resistin are positively associated with RA disease activity,2,3 but while Resistin showed a limited correlation with other inflammatory cytokines, Leptin showed a positive association with IL-1β. It must be remembered though that in some studies,2 the observations have been longitudinal while this study made a single, transversal measurement. In contrast to other authors’ publications, we found no correlation between Leptin and IL-63 or IL-17,2 possibly because of differences in the origin of samples (synovial fluid versus peripheral blood) and design (transversal versus longitudinal). Otherwise, in support of the present results, studies with human pancreatic islet or cord blood T-cells showed that in vitro stimulation by Leptin was able to induce IL-1β secretion.11
Conversely, Adiponectin was not associated to clinical disease activity; however, it did show a differential association with IL-1β and TNFα levels: a positive correlation with IL-1β and a negative one with TNFα. Previous studies have confirmed the antagonistic relationship between TNFα and Adiponectin12 and have observed a synergistic effect of Adiponectin and IL-1β in promoting pro-inflamatory cytokine secretion.13 However there is still controversy regarding its exact role in RA inflammation and it has a major metabolic role in insulin sensitivity, which might influence the characteristics of each patient depending on other factors such as adiposity. Although Adipocytokines depend on white fat tissue for secretion, in this study BMI did not play a significant role in cytokine/adipocytokine levels when controlled for in the correlation analysis. Gender, age, time since onset or ESR were also controlled for and changes in the cytokine or adipocytokine levels were also independent of them.
As seen by other authors,14 circulating levels of IL-6 and IL-17 from moderate/high activity (DAS28 >3.5) RA patients were significantly higher than those from patients in remission or with low disease activity. Additionally, there was a disparity in the circulating levels of TNFα, IL-1β, IL-6 and IL-17: TNFα was positively associated with IL-17, but a negative correlation was observed with IL-1β and no association was found with IL-6. In support of our results, previous studies have observed an antagonistic relation between TNFα and IL-1β, a positive association between IL-1β and IL-6 and a disparity between TNFα and IL-6,15 probably reflecting a complex relationship between circulating cytokines in RA patients.
The present study, however, has several limitations, including a small sample size, the heterogeneity of DMARD treatment and the limitations in treatment. Due to the fact that patients had no access to biologic therapy, and that toxicity due to Methotrexate was frequently seen using doses over 15mg/week, partial or complete remission was not achieved in many cases, a factor that also plays a role in the results obtained. However, since all of the patients were followed and treated by only one clinician, the clinical criteria employed for their evaluation was homogeneous. Another limitation was that the C-reactive protein in our patients, which has an important relationship to some of the evaluated cytokines, was not heterogeneously measured and therefore was not included in the analysis.
In conclusion, circulating Leptin, Resistin, IL-6 and IL-17 levels positively correlated with the clinical activity of RA patients in a BMI-independent way. Due to the many inflammatory and metabolic pathways involved, these complex relationships warrant further study.
Ethical disclosuresProtection of human and animal subjectsThe authors state that the procedures conformed to the ethical standards of human experimentation committee responsible and according to the World Medical Association Declaration of Helsinki.
Confidentiality of dataThe authors declare that this article does not appear patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors declare they do not have any conflicts of interest.
The authors wish to thank MMM. José Iván Martínez Rivera for performing the multivariable statistical analysis. This work was supported by the Consejo Nacional de Ciencia y Tecnologia (CONACYT, CB2010-01-155392). C.BR.B received a stipend from CONACYT during the study.