Primary Raynaud's phenomenon (PRP) manifests as episodes of transient spasms of peripheral blood vessels. To elucidate the clinical clues and laboratory characteristics will facilitate the identification of PRP.
MethodsA retrospective data collection of clinical and laboratory characteristics of 58 children with PRP was performed between January 2007 and December 2016.
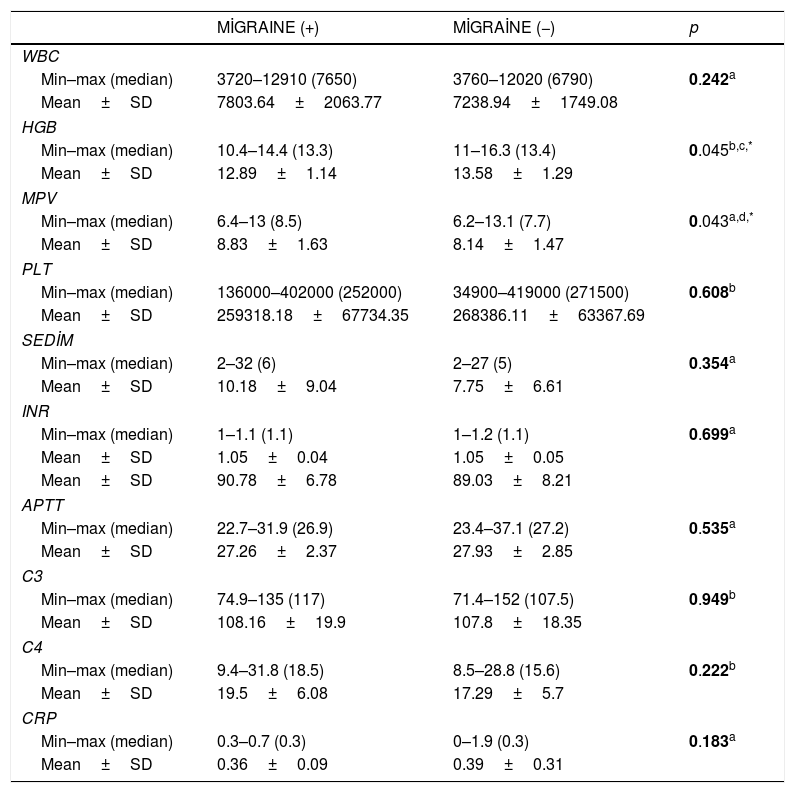
ResultsA positive ANA test at lower titers <1:100 was detected in 24.1% of the patients. There was a significant relationship between presence of ANA positivity and migraine in female patients with PRP (p=0.01; p=0.020 respectively). The most common accompanying disorder was migraine which was detected in 37.9% of all patients with PRP. Hemoglobin and serum ferritin levels were significantly lower in PRP patients with migraine (p=0.045; p<0.05, respectively). Additionally, the mean platelet volume (MPV) measurements were significantly higher in patients with migraine compared to those without migraine (p=0.045; p<0.05 respectively).
DiscussionThere is limited data concerning childhood PRP. For the first time we showed a high frequency of migraine in childhood PRP. Anemia and high MPV could be the underlying triggering factors of these two episodic diseases.
El fenómeno de Raynaud primario (PRP, por sus siglas en inglés) se manifiesta como episodios de vasoespasmos transitorios de vasos sanguíneos periféricos. Elucidar las características clínicas y de laboratorio facilitará la identificación del PRP, así como las enfermedades acompañantes.
MétodosSe realizó una un estudio retrospectivo de datos clínicos, de laboratorio y de tratamiento en 58 niños con PRP entre enero de 2007 y diciembre de 2016.
ResultadosSe detectó una prueba de ANA positiva en títulos inferiores (<1:100) en el 24,1% de nuestros pacientes. Se encontró correlación estadística entre la positividad de anticuerpos antinucleares y la presencia de migraña en las mujeres con PRP (p=0,01; p=0,020, respectivamente, prueba exacta de Fisher, prueba de corrección de continuidad de Yates). El trastorno más común fue la presencia de migraña en el 37,9% (n=22) asociado con PRP. Los niveles de hemoglobina fueron significativamente más bajos asociados con un bajo nivel de ferritina sérica en los casos de PRP con migraña (p=0,045; p<0,05). Además, las mediciones del volumen plaquetario medio (VPM) fueron significativamente mayores en los casos con migraña en comparación con aquellos sin migraña (p=0,045; p<0,05).
DiscusiónExisten datos muy limitados sobre el PRP infantil. Hemos presentado por primera vez la asociación de PRP infantil y migraña en un estudio retrospectivo. La anemia y el VPM alto podrían formar parte de los factores predisponentes de ambas enfermedades episódicas.
Raynaud's phenomenon (PRP) is a vascular disorder characterized by episodic and reversible attacks of vasospasm with paleness of the extremities, followed by cyanosis and hyperemia. Arteriovenous anastomoses are richly innervated by sympathetic nerves and are normally exposed to increased sympathetic vasoconstriction under resting thermoneutral conditions and when sympathetic activity is increased during stress or exposure to cold. Prevalence of PRP in the general population ranges 5–20%, while in between 12 and 15 year old children this rate is 15%.1–4 Females are much more susceptible to RP than males. Hormonal factors may account for the higher prevalence of RP in females. Primary RP occurs without any underlying disease and is considered a benign condition that is mostly seen during childhood. In contrast, secondary RP is associated with other diseases; mainly connective tissue diseases. The clinical absence of any underlying disease, negative autoimmune serology and serum inflammatory markers associated with normal capillaroscopy findings lead to the diagnosis of PRP.
A significant increase has been reported in the prevalence of migraine in adult patients with PRP and vice versa.5,6 Vascular endothelial dysfunction has been demonstrated in patients with migraine, which might explain the increased prevalence of RP in patients with migraine.
The avoidance of cold temperatures is the best method to prevent an episode of Raynaud's phenomenon. Keeping the whole body warm by wearing loose-fitting clothing, stockings, headwear and gloves in cold weather is a key strategy.7 A variety of factors can potentially aggravate the disorder and should be avoided, including smoking, caffeine, the use of sympathomimetic drugs and nonselective beta-blockers. Traditional pharmacological drugs alleviate RP symptoms by reducing vasoconstriction, inducing vasodilatory effect such as topical nitrate, calcium-channel blockers.
In this study, we aim to elucidate the clinical and laboratory characteristics that will facilitate the identification of PRP as well as the concomitant diseases.
Material and methodsA total of 58 cases followed up at the department of Pediatric Immunology and Rheumatology, Uludag University Faculty of Medicine between January 2007 and December 2015 were enrolled in our study. International consensus criteria for the diagnosis of Raynaud's Phenomenon were used to diagnose patients with RP in our study.8 A characteristic “triphasic” color pattern, (pallor, cyanosis, rubor) as well as numbness and swelling were the presenting symptoms of the patients. Patients older than 18 years old and with systemic autoimmune rheumatic diseases were excluded. Laboratory evaluation included hemogram, testing for acute phase proteins (erythrocyte sedimentation rate, serum amyloid A and C-reactive protein), autoantibodies (antinuclear antibodies, rheumatoid factor, anti-cardiolipin IgM and IgG), blood clotting markers (prothrombin time, activated partial thromboplastin time and fibrinogen). Demographic data, clinical and laboratory findings of our patients with PRP were documented from patient files retrospectively.
NCSS 2007 (Number Cruncher Statistical System) & PASS (Power Analysis and Sample Size) and 2008 Statistical Software (NCSS LLC, Kaysville, UT, USA) programs were used for the statistical analysis. During the evaluation of the data obtained from the study, descriptive statistical methods of mean, standard deviation, frequency, and ratio values were used in the tables. Kolmogorov Smirnov test and box plot plots were used for the normal distribution suitability of the quantitative data. Student t test was used for intergroup comparison of normal distribution variables, and Mann Whitney U test was used for intergroup comparisons of variables without normal distribution. In the comparison of qualitative data, Yates Continuity Correction, Fischer's Exact test and Fischer Freeman Halton test were used. The confidence interval was determined as 95% and a p value <0.05 was accepted to be significant. The IFA (indirect fluorescent antibody) method was used as the main screening test for ANA.
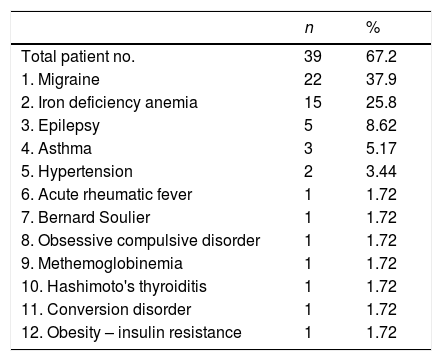
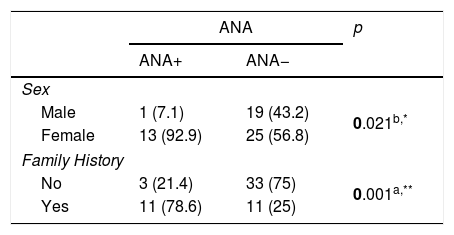
ResultsWe evaluated 20 (34.5%) male and 38 (65.5%) female patients with PRP in this study. Their ages ranged between 6 and 21 years; the mean age of the patients at diagnosis was 15.74±2.88 years. Biphasic change in the skin color of the digits was observed in 65.5% (n=38) whereas in 34.5% (n=20) of the patients had triphasic color change. While being exposed to cold took first place in the investigation of the triggering factors, stress and exercise were in the following order (72.4% and 29.3%), respectively. Family history was present in 37.9% (n=22) of the patients. 67.5% of the patients with PRP had an accompanying disease, where migraine is the most frequent of them (37.9%) (Table 1). The diagnosis of childhood migraine was made according to the International classification of Headache Disorders-II (ICHD-II criteria).9 24.1% (n=14) of our patients have positive ANA test at lower titers (<1:100). ANA positivity and presence of migraine were statistically higher in female patients with PRP. The frequency of PRP in family members was found to be statistically high in ANA positive cases. 11 of 14 patients with ANA positivities had a Raynaud phenomenic story in their family members (Table 2).
Accompanying diseases in patients with Raynaud's phenomenon.
| n | % | |
|---|---|---|
| Total patient no. | 39 | 67.2 |
| 1. Migraine | 22 | 37.9 |
| 2. Iron deficiency anemia | 15 | 25.8 |
| 3. Epilepsy | 5 | 8.62 |
| 4. Asthma | 3 | 5.17 |
| 5. Hypertension | 2 | 3.44 |
| 6. Acute rheumatic fever | 1 | 1.72 |
| 7. Bernard Soulier | 1 | 1.72 |
| 8. Obsessive compulsive disorder | 1 | 1.72 |
| 9. Methemoglobinemia | 1 | 1.72 |
| 10. Hashimoto's thyroiditis | 1 | 1.72 |
| 11. Conversion disorder | 1 | 1.72 |
| 12. Obesity – insulin resistance | 1 | 1.72 |
According to the ANA variable, the evaluation of the gender of the patients and the family history.
| ANA | p | ||
|---|---|---|---|
| ANA+ | ANA− | ||
| Sex | |||
| Male | 1 (7.1) | 19 (43.2) | 0.021b,* |
| Female | 13 (92.9) | 25 (56.8) | |
| Family History | |||
| No | 3 (21.4) | 33 (75) | 0.001a,** |
| Yes | 11 (78.6) | 11 (25) | |
Hemoglobin levels were significantly lower associated with low serum ferritin level in PRP patients with migraine. Additionally, the mean platelet volume (MPV) measurements were significantly higher in patients with migraine compared to those without migraine (Table 3).
Comparison of laboratory measurements of cases according to presence of migraine.
| MİGRAINE (+) | MİGRAİNE (−) | p | |
|---|---|---|---|
| WBC | |||
| Min–max (median) | 3720–12910 (7650) | 3760–12020 (6790) | 0.242a |
| Mean±SD | 7803.64±2063.77 | 7238.94±1749.08 | |
| HGB | |||
| Min–max (median) | 10.4–14.4 (13.3) | 11–16.3 (13.4) | 0.045b,c,* |
| Mean±SD | 12.89±1.14 | 13.58±1.29 | |
| MPV | |||
| Min–max (median) | 6.4–13 (8.5) | 6.2–13.1 (7.7) | 0.043a,d,* |
| Mean±SD | 8.83±1.63 | 8.14±1.47 | |
| PLT | |||
| Min–max (median) | 136000–402000 (252000) | 34900–419000 (271500) | 0.608b |
| Mean±SD | 259318.18±67734.35 | 268386.11±63367.69 | |
| SEDİM | |||
| Min–max (median) | 2–32 (6) | 2–27 (5) | 0.354a |
| Mean±SD | 10.18±9.04 | 7.75±6.61 | |
| INR | |||
| Min–max (median) | 1–1.1 (1.1) | 1–1.2 (1.1) | 0.699a |
| Mean±SD | 1.05±0.04 | 1.05±0.05 | |
| Mean±SD | 90.78±6.78 | 89.03±8.21 | |
| APTT | |||
| Min–max (median) | 22.7–31.9 (26.9) | 23.4–37.1 (27.2) | 0.535a |
| Mean±SD | 27.26±2.37 | 27.93±2.85 | |
| C3 | |||
| Min–max (median) | 74.9–135 (117) | 71.4–152 (107.5) | 0.949b |
| Mean±SD | 108.16±19.9 | 107.8±18.35 | |
| C4 | |||
| Min–max (median) | 9.4–31.8 (18.5) | 8.5–28.8 (15.6) | 0.222b |
| Mean±SD | 19.5±6.08 | 17.29±5.7 | |
| CRP | |||
| Min–max (median) | 0.3–0.7 (0.3) | 0–1.9 (0.3) | 0.183a |
| Mean±SD | 0.36±0.09 | 0.39±0.31 | |
Nitroglycerin patch band was prescribed for 44.8% (n=26) of the patients and the clinical symptoms in 50% of them were improved. Calcium channel blockers was effective 78% of 32 patients were on this therapy.
DiscussionPrimary RP is more common in children than secondary RP. The onset of the symptoms of PRP occurs during adolescence period. Considering the limited number of studies made on PRP in children, we found out that girls are more predisposed to develop PRP and the onset occurs during the changes observed around menarche, due to the influence of sex hormones. Similarly, our study revealed that the mean age of symptom onset was around adolescence. The largest cohort published in 2003, involving 123 children with Raynaud's phenomenon, demonstrated that exposure to cold was the major trigger in majority of the cases (around 70%) but, in 10%, no trigger was identified.10 Similarly, cold was the major trigger of attacks in our patients, followed by stress. Falcini et al. performed a retrospective multi-center data collection of 94 Caucasian children and adolescents with RP who were observed for a period of 3 years. In conclusion, the only parameters significantly associated with the evolution of RP toward a CTD were ANA positivity.11 In our study, between 2007 and 2016, patients were diagnosed with PRP at different times. If an underlying cause of secondary connective tissue disease was detected, it was removed without further study. Since the initial diagnosis, 14 patients with ANA positivity of 1/100 titer were closely followed. However, symptoms of secondary raynaud phenomenia or laboratory findings did not develop. We attributed this to the detection of ANA positivity at very low titers and to the ANA positivity seen in healthy populations in the Mediterranean and Middle Eastern countries. We must emphasize that the antinuclear antibody, which is a laboratory parameter with a high positivity rate especially in the Mediterranean and Middle East countries, will never be sufficient in itself. Clinical correlation is the most important criterion in diagnosis. It is necessary for patients with RP to undergo a complete investigation to exclude an underlying secondary cause and long-term follow-up is essential to identify new findings that may develop with the time.
Family history of PRP is one of the major risk factors of PRP. A retrospective study involving 900 patients showed a positive family history of RP in 3% of affected patients.12 In ANA positive patient group for our study, we found a high frequency of PRP in family members of patients (p=0.001) (Table 2).
Migraine and RP are both independently common disorders in general population. PRP association with migraine is due to the similarity in the underlying pathogenesis of both diseases. It was considered that functional diseases of small-vessels might be the underlying cause of RP clinical features and may contribute to the cerebrovascular mechanism causing migraine. There are many studies in the adult patient population related to the association of migraine and Raynaud phenomenia.6,13–16 Also there is a metaanalysis for all ages that shows the prevalence and incidence of PRP in different countries. Female gender, positive family history, smoking and migraines were found to be the major risk factors for PRP.17 However, there is limited data concerning about childhood PRP and migraine. In this study, we first showed a high frequency of migraine (37.9% of all patients) in childhood RP (v 1). There is only one case report on RP and migraine association in children.18
RP has been studied by several authors from a rheological point of view.19–21 In 29 primary RP patients, Ziegler et al. found higher fibrinogen levels in patients than in healthy controls, for only the male gender.22 Spengler et al. observed increased fibrinogen and erythrocyte aggregation in RP patients with a pathological capillary pattern.23 Picart et al. found an increased erythrocyte sedimentation rate and elevated fibrinogen levels in secondary RP24 Vaya et al. studied variables in the laboratory parameters between primary, secondary Raynaud phenomenon patients and healthy control group.25 As a result of the study, blood sugar, plasma viscosity, IgG, IgA, cholesterol levels were found higher in the group of patients with secondary Raynaud phenomenon than in healthy control group. There was no significant difference between the PRP group and the healthy control group. Our study included pediatric population with PRP. When we compared the patients in this population with the presence of accompanying migraine, we obtained interesting results. The presence of anemia in PRP children with migraine compared to those without migraine was significantly higher in our study (Table 3). It suggests that anemia could be a triggering factor for the development of migraine in cases with PRP. Therefore, we speculated that correction of anemia might reduce the frequency and severity of both migraine and PRP attacks in these patients. The elevation of mean platelet volume (MPV) has been reported in various diseases, including idiopathic thrombocytopenic purpura, Bernard Soulier disease, May Haggle anomaly, myeloproliferative diseases, and vasculitis.26–29 In our study, we identified a statistically significant higher MPV levels in PRP patients accompanied with migraine compared to those without migraine (Table 3). Increased MPV may be one of the factors playing a role in the pathogenesis of both migraine and PRP characterized by episodic vasoconstriction.
In conclusion, we first showed the association of childhood PRP and migraine in a retrospective study.
Anemia and high MPV might be among the predisposing factors of these episodic diseases. Further studies are needed to disclose the risk factors as far as children having both PRP and migraine attacks are concerned. Besides taking precautions, we prescribed calcium blockers and topical vasodilators for the treatment of our patients.
FundingThe authors declare that they have no source of funding for the performance of this study.
Conflict of interestsThe authors declare they have no conflicts of interest.