The highest incidence of death in systemic sclerosis due to pulmonary disease raises the need for early detection and treatment. The study aim is the assessment of interstitial pulmonary disease by Multi Detector High Resolution CT (MDCT) and finds its relationship with the other disease parameters and the Pulmonary Function tests (PFT).
Patients and methodsA prospective cross-sectional study was performed in Assiut University Hospitals from May 2018 to January 2020 and included 62 consecutive SSc female patients. Demographic, clinical, Laboratory, PFT and MDCT assessment were conducted for all participants.
ResultsThe coarseness of fibrosis was 8.32 (range 0.0–17), the average proportion of ground-glass opacification was 28.3% (range, 0.0%–75%). Honey-comb pattern was seen in (52.5%). Mean Extent of disease was 46.25±3.7 (range 5–81). Restrictive deficit found in 42 patients. Significant relation was found between the extent of disease and the percentage predicted FVC (r=0.373, p 0.018) and FEV1/FVC (r=0.593, p 0.000) and coarseness of fibrosis and proportion of ground glass opacification correlated inversely with VC (r=−0.385, p=0.014, r=−0.376, p=0.017 respectively), Rayanud's phenomena, modified Rodnan Skin Score and Medsger's general are positively correlated with MDCT disease extent.
ConclusionScoring of systemic sclerosis (SSc) related interstitial lung disease (SSc-ILD) could be applicable as one of the important tools for disease assessment.
La mayor incidencia de muerte en la esclerosis sistémica por enfermedad pulmonar plantea la necesidad de una detección y un tratamiento precoces. El objetivo del estudio es la evaluación de la enfermedad pulmonar intersticial mediante TC de alta resolución multidetector (TCMD) y encuentra su relación con otros parámetros de la enfermedad y con pruebas de funcionamiento pulmonar (PFP).
Pacientes y métodosSe realizó un estudio transversal prospectivo en los hospitales universitarios de Assiut desde mayo de 2018 hasta enero de 2020 que incluyó 62 pacientes femeninas de esclerosis sistémica consecutivas. Se realizaron evaluaciones demográficas, clínicas, de laboratorio, PFP y TCMD para todos los participantes.
ResultadosLa aspereza de la fibrosis fue de 8,32 (rango 0,0-17) y la proporción promedio de opacificación en vidrio esmerilado fue del 28,3% (rango 0,0-75%). Se observó un patrón de panal de miel en el 52,5%. La extensión media de la enfermedad fue de 46,25±3,7 (rango 5-81). Se encontró déficit restrictivo en 42 pacientes. Se encontró una relación significativa entre la extensión de la enfermedad y el porcentaje predicho de capacidad vital forzada (CVF) (r=0,373, p=0,018) y FEV1/CVF (r=0,593, p=0,000) y la aspereza de la fibrosis y la proporción de opacificación en vidrio esmerilado se correlacionaron inversamente con la capacidad vital (r=−0,385, p=0,014; r=−0,376, p=0,017, respectivamente), los fenómenos de Rayanud, m Rodnan Skin Score y Medsger general se correlacionan positivamente con la extensión de la enfermedad por TCMD.
ConclusiónLa puntuación de la enfermedad pulmonar intersticial relacionada con la esclerosis sistémica podría ser aplicable como una de las herramientas importantes para la evaluación de la enfermedad.
Systemic sclerosis (SSc) is well known as a multifactorial systemic autoimmune disease with structural and functional damage of the microvasculature1 this could happen in the form of endothelial dysfunction with small-vessel vasculopathy, immunologic abnormalities, fibrosis and excessive collagen production.2 Systemic sclerosis is classified into diffuse SSc (dcSSc) and limited SSc (lcSSc) types according to the pattern of skin involvement.3
Pulmonary affection in patients with SSc is a common co-morbidity, it is classified into two main groups, (1) primary pulmonary disease with lung parenchyma involvement and pulmonary hypertension, (2) secondary pulmonary disease as a complication of airway illness with bronco-aspiration secondary to gastro-esophageal reflux, toxicity due to medications, and infections.4
Interstitial lung disease (ILD) is found in some studies more common in diffuse SSc than limited type.1,5 Pulmonary hypertension (PH) and ILD, are the most common cause of morbidity and mortality in SSc patients.1 Recognition of lung involvement is essential for the care of these patients.2 Interstitial abnormalities on high-resolution computed tomography (HRCT) could be detected in about 90% of SSc patients,6 while 40–75% will have changes in pulmonary function tests (PFTs).7 Previous studies of the course of systemic sclerosis disease have found some predicting factors for ILD in SSc for example; autoantibodies such as antitopoisomerase, age at the diagnosis of SSc, cardiac diseases, elevated Rodnan Skin Score, elevated creatinine, forced vital capacity (FVC) less than 65%, carbon monoxide diffusion capacity (DLCO) less than or equal to 55%, and the dcSSc.8–10
Material and methodsA prospective cross-sectional study was performed from May 2018 to January 2020, it included 62 consecutive SSc female patients. The inclusion criteria were the following: The SSc patients fulfilled the 2013 (ACR/EULAR) classification criteria for scleroderma,11 and were further sub-classified to have diffuse (dcSSc) or limited (lcSSc) cutaneous subset of SSc,3 age at disease onset after 16 years old, and patients use stable medication in the last 3 months. Exclusion criteria included; mixed connective or autoimmune tissue diseases overlapping with SSc, recent or current respiratory infection, uncontrolled congestive heart failure, severe pulmonary hypertension requiring specific treatment, history of allergic alveolitis or asthma, exposure to organic dusts or clinically significant abnormalities other than interstitial lung disease identified on chest radiography or on HRCT.
The study was approved by the university ethical committee Ass-16-17100179m.
All patients signed informed consent to join the study
MethodsSSc patients underwent: demographic data, clinical assessment, pulmonary function tests (PFT), high resolution MDCT, and laboratory assessment.
Clinical assessment included; disease history and duration, modified Rodnan Skin Score (mRSS), revised Medsger's SSc severity scale which consists of nine organ systems with a score from 0 to 4,12 electrocardiogram (ECG, estimation of pulmonary artery systolic pressure (PASP)), and left ventricular ejection fraction (LVEF) with Echo Doppler.
PFT were performed (MasterScreen Diffusion, Jaeger, Wuerzburg, Germany) included spirometry, lung volume determination by the helium dilution technique, and CO diffusion lung capacity measurement by the single-breath method, according to the American Thoracic Society13 guidelines. The obtained values were expressed as percentages of normal predicted values. Pulmonary function was considered abnormal when total lung capacity (TLC) and/or forced vital capacity (FVC) and/or carbon monoxide lung diffusion (DLCO) were less than 80% of predicted values. Spirometric parameters included forced expiratory volume in 1s (FEV1), forced vital capacity (FVC) and FEV1/FVC, vital capacity (VC) were expressed as a percentage of predicted value (% pred. values) based on height, age, gender and ethnic origin.
High-resolution multidetector CT (MDCT) scans were obtained on 16 rows MDCT (GE medical systems) scanner with 0.75 collimation, 0.5s gantry rotation time, 140kVp, and 120mAs during maximum inspiration with the patient supine and scan was performed from lung apex to diaphragm. All images were reconstructed with a slice thickness of 1mm and a reconstruction interval of 1mm with high-frequency reconstruction kernel. Images were reconstructed with a high spatial frequency algorithm for lung analysis and with a standard soft-tissue algorithm for mediastinal evaluation. Images were viewed at lung (window width 1600 HU, window level 600 HU) and mediastinal (window width 350 HU, window level 50 HU) window settings. No intravenous contrast material was used.
Interpretation of high-resolution MDCT imagesThe high-resolution MDCT features were quantified visually using quantitative and semi-quantitative scoring in blinded manner similar to that reported by Wells et al.,14 and Desai et al.15 Total disease extent was estimated to the nearest 5%. Ground glass opacification (GGO) and reticular pattern extents were estimated as proportion of total disease extent. Honey-combing (HCs) was estimated as well. The extent of pulmonary disease was evaluated in 5 levels of the lungs, as follows: 1, origin of great vessels; 2, Carina; 3, pulmonary venous confluence; 4, between levels 3 and 5; and 5, 1cm above the right hemidiaphragm. The coarseness of fibrosis was graded as follows: 0, ground-glass opacification alone; 1, fine intra-lobular fibrosis; 2, microcystic reticular pattern comprising air spaces smaller than or equal to 4mm in diameter; and 3, a macrocytic reticular pattern comprising air spaces larger than 4mm in diameter.
The CT findings were graded as follows: grade 1, predominant ground-glass opacification; grade 2, equal proportions of ground-glass opacification and a reticular pattern, and grade 3, predominant reticular pattern.15
Statistical analysisData were expressed as mean and standard deviation for continuous variables and as number and percentage for categorical variables. Statistical analysis was performed using the SPSS package (SPSS Inc, Chicago, U.S.A.) (version 20). The t-test and one-way ANOVA test were applied for comparisons among variables. The relationships between MDCT scores and pulmonary function parameters were examined using Pearson's correlation. A p value of than 0.05 was considered statistically significant.
ResultsDemographic dataThe mean (±SD) age was 38.45±9.5 years. 63% were positive ANA, 61% were anti SCL70 positive and 29% were ACA positive. Forty-one patients (66%) had limited SSc while 21(34%) had diffuse SSc. Raynaud's phenomena were present in 51 (82%), digital ulcers in 37 (60), 22 (35.5%) had telangiectasia, 35 (56.5%) had calcinosis. Dyspnea and heart burn were present in 46 (74%) and dysphagia occurred in 47(74.6%). The mean and standard deviation of the modified Rodnan Skin Score (MRSS) was 24.9±9.3.
Demographic data of all patients were summarized in Table 1.
Demographic data.
| Limited SSc (lcSSc) | Diffuse SSc (dcSSc) | p value | |
|---|---|---|---|
| Number of patients | 41 (66%) | 21 (34%) | |
| Age | 37.15+9.38 | 41.15+9.56 | 0.216 |
| Disease duration in years | 6.22+4.16 | 7.38+5.45 | 0.460 |
| Raynaud's phenomena | 25 (92.5%) | 12 (92.3%) | 0.974 |
| ESR | 23.63+16.74 | 26.23+19.72 | 0.666 |
| Renal affection | 5 (12%) | 5 (23.1%) | 0.962 |
| Cardiac affection | 3 (7.4%) | 0 (0%) | 0.314 |
| Pulmonary affection | 26 (63%) | 8 (38%) | 0.145 |
| Treatment | |||
| Steroids | 21 (77.8%) | 13 (100%) | |
| MTX | 13 (48.1%) | 7 (53.8%) | |
| AZA | 9 (33.3%) | 4 (30.7%) | |
| CYC | 0 (0%) | 3 (23.1%) | |
| Epilate retard | 10 (37.1%) | 4 (30.7) | |
| Capiton | 3 (11.1%) | 3 (23.1%) | |
| PFTs | |||
| FEV1 | 85.44+18.98 | 93.77+20.54 | 0.213 |
| FEV1/FVC | 106.7+6.92 | 106.85+6.59 | 0.951 |
| FVC | 61.22+20.49 | 70.69+23.81 | 0.202 |
| VC | 71.15+13.12 | 84.62+24.25 | 0.028* |
ESR; erythrocyte sedimentation rate, MTX; methotrexate, AZA; azathioprine, CYC; cyclosporine, FEV1; forced expiratory volume in 1s, FVC; forced vital capacity, VC; vital capacity.
The average of coarseness of fibrosis was 8.32 (range, 0.0–17), the average proportion of ground-glass opacification was 28.3% (range, 0.0%–75%). The mean extent of disease was 46.25±3.7 (range 5–81) HR-MDCT findings were summarized in Table 2.
HRCT showed that lung reticular pattern was the most common lung abnormality, which was found in 31(50%) patients. The GGO was found in 21 (33%) patients, mixed pattern was found in 11 patients (17.5%), and honey-comb (HCs) pattern was seen in 33 (53%) patients. Patients with positive anti-topoisomerase antibodies showed more sever pattern than negative patients.
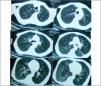
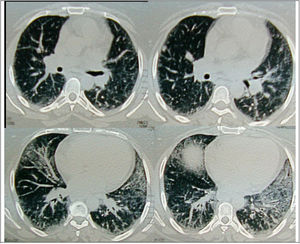
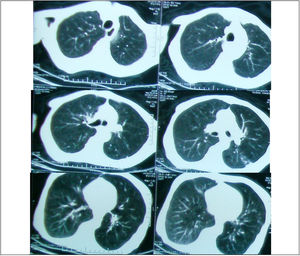
Regarding the regional distribution of abnormalities, reticular pattern and HC were the most common in the lower lung zones (Figs. 1 and 2).
Axial HRCT images at level 3 and 4 in a 38 year-old female show mixed reticular and GGO patterns as septal thickening in lung bases and limited patchy areas of GGO. This patient had FVC 61% of predicted value (mild restriction pulmonary impairment). A predominant reticular pattern and a coarseness grade of 3 were assigned.
Respiratory restrictive pattern was found in 47 (75%) patients (mild to moderate in 29 patients and severe in 18 patients), the rest 15 patients had normal PFTs.
A significant difference was found between subtypes of SSc and vital capacity VC (p=0.028) but other spirometric parameters showed no significant differences (p>0.05). The coarseness of fibrosis shows significant difference between subtypes of SSc (p=0.025) but the proportion of ground-glass opacification, extent of disease and honeycomb did not differ significantly between lcSSc and dcSSc (p>0.05) (Table 3).
Comparison between SSc subtypes and MDCT parameters.
| HR-MDCT parameters | lcSSc (n=41) | dcSSc (n=21) | p value |
|---|---|---|---|
| Extent of disease (%) | 46.30 | 54.08 | 0.652 |
| Proportion of ground glass opacification (%) | 29.5 | 22.62 | 0.792 |
| Coarseness of fibrosis (%) | 8.19 | 6.31 | 0.026* |
| Presence of honey combing | 55.6 | 46.2% | 0.577 |
| Overall grade | |||
| 1 | 9 | 4 | |
| 2 | 5 | 2 | |
| 3 | 13 | 7 | 0.946 |
* p value is significant.
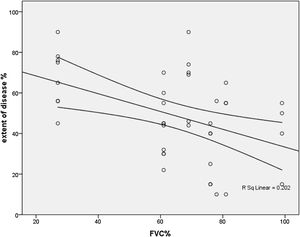
Thirty- seven (60%) patients had FVC<70% of predicted. An inverse correlation was found among extent and FVC (r=−448, p=0.004), FEV1/FVC (r=−380, p=0.015) and VC (r=353, p=0.025) (Fig. 3).
Disease duration was positively and significantly correlated with extent of disease (r=0.52, r=0.05), Medications’ dose and duration did not show any significant correlations with the pulmonary assessments.
Rodnan skin score was positively and significantly correlated with extent of disease (r=0.59, p=0.001), with honey comb (r=0.37, p=0.018), and negatively correlated with FVC (r=−.56, p=0.01).
The correlation of both Medsger's General and Medsger's kidney were positively significant with the coarse pattern of MDCT (r=0.34, p=0.03) and (r=0.54, p=0.02) respectively, while other Medsger's scales did not show any significant relations.
All patients known to have signs of ILD by MDCT examination in this study were having Raynaud's phenomena as well.
DiscussionThe importance of studying the interstitial lung disease in systemic sclerosis patients (SSc-ILD) is increasing, as it is considered with the pulmonary hypertension as the most leading causes of disease related death in SSc patients.16 As new therapies targeting these pulmonary conditions emerge, early recognition of lung involvement is essential for the care of these patients
The prevalence of (ILD) in SSc patients varies by the diagnostic method. Some interesting studies examined series of autopsies, and found100% of the SSc cases presented with ILD.17,18 And with the use of high resolution computed tomography (HRCT), this disease was detected in up to 90% of cases,2,6,19 while the pulmonary function tests indicated ILD in 40–75% of cases.2
HRCT imaging provides means of accurately characterizing the nature and extent of lung parenchymal changes. SSc-ILD typically manifests on HRCT as predominantly ground glass appearance (GGO) with a variable degree of pulmonary fibrosis. Honeycomb cystic change were detected in 11–37% of patients with SSc-ILD.20 Thus, HRCT can be used to depict early SSc-ILD. Hence, in the current study, reticular pattern was the most common MDCT patterns of lung disease in 50% of SSc patients with the GGO (with or without fibrosis) and HC were observed in 33% and 52.5% patients respectively. This was in agreement with Wangkaew et al.21 who observed predominant reticular pattern, GGO and HC in his patients.
In the current study, we used a quantitative scoring method that was proposed by Wells et al.14 in order to estimate the extent of disease by HR-MDCT. The extent of lung involvement on HRCT images (reticular, GGO, and/or HCs) is a more direct and precise indicator of the severity of the underlying pathological process.22–25 Also, it is a predictor of decline in forced vital capacity (FVC) in SSc-ILD. A significant correlation found between the extent of disease and PFTs (p<0.05). Although using different scoring systems, this is consistent with those of previous studies.26,27
Notably, in this study, 15 of 62 patients with normal FVC value had occult lung fibrosis depicted on HRCT. This is supported by Suliman et al.28 who concluded that the absence of pulmonary symptoms does not exclude lung fibrosis in patients with normal FVC and low sensitivity of PFTs to detect early ILD compared with HRCT.
Significant differences were observed in the current study, with respect to HRCT-coarseness of fibrosis score among subtypes of SSc that it was worse in limited than diffuse subtype (8.19 vs. 6.31, p<0.026) and it was in agreement with Scleroderma lung study (SLS) (23) who stated that the baseline HRCT total fibrosis score was significantly higher (worse) in patients with lcSSc (p=0.046) on the other hand, Patiwetwitoon et al.26 found that the HRCT scores were comparable in both subtypes of SSc.
Scleroderma Lung Study (SLS)29 reported that “there were no significant differences between lcSSc and dcSSc patients related to the frequency of alveolitis (GGO) by HRCT or the average values of pulmonary functions”. This was similar to the current study observation and in harmony with the other authors.30–32
Furthermore, Clements29 added that approximately 40% of the patients enrolled into the SLS with dyspnea, restrictive lung disease and evidence of alveolar inflammation on Broncho-alveolar lavage (BAL) or HRCT had lcSSc. Therefore, the assumption that SSc-related ILD would be more common and severe in patients with diffuse SSc was discredited. So all SSc patients should be evaluated carefully for lung involvement irrespective of disease extent.16,30
Approximately 40% of patients with SSc develop moderate to severe restrictive lung disease16 it as in accordance with the current study that a restrictive defect found in 75% of patients.
The association of Raynaud's phenomena with positive MDCT in our study patients could help in the explanation of the ILD pathogenesis in SSc disease as a part of abnormal interactions between endothelial cells, lymphocytes/monocytes and fibroblasts with subsequent an excess production of extracellular matrix by fibroblasts in the setting of tissue hypoxia and vascular hyperreactivity.19,33
During the assessment of SSc outcome measures, the application of m RSS is approved as a measuring tool for the degree of skin thickening as the hallmark of SSc and as a primary outcome measure of the therapy.34 The presence of the positive and significant correlation of the m RSS with the extent of disease (r=0.59, p=0.001), and with the honey comb (r=0.37, p=0.018), and negative correlation with FVC (r=−.56, p=0.01) is a good indicator of the importance of investigating the pulmonary affection is SSc patients even if they not yet developed pulmonary symptoms.
Both Medsger's General and Medsger's kidney were positively significant correlated with the coarse pattern of MDCT (r=0.34, p=0.03) and HC (r=0.54, p=0.02) respectively and that also walks in the same way of the strong association of pulmonary involvement with the different disease parameters.
Limitation of the study: Firstly, our study focused only on quantification of ILD extent. The mean diameter of pulmonary artery was not assessed in the current study. Secondly, lacking the correlation of HRCT scan with long-term outcome, in additionto that, the capillaroscopic examination was not performed for the participants.
Thus, cross-sectional and multicenter study is recommended in our locality.
ConclusionScoring of SSc-ILD is recommended to be applicable as a routine evaluation of patients with SSc coarseness of fibrosis score was worse in lcSSc than in dcSSc. And also, there is a good correlation between the extent of HRCT findings with different SSc diseases assessment parameters.
Conflict of interestAll authors have no conflict of interest.
We would like to thank Professor Mahmoud Elashrey, Internal Medicine Department, Assiut University Hospitals, for his enthusiastic support during this research study. This study has not received any institutional or governmental fund support, and no funded grants.