Smoking is one of the few modifiable risk factors associated with the development of rheumatoid arthritis (RA). Most published data are over 10 years old, and none included Mediterranean populations. We therefore took advantage of primary care routinely collected data to study the association between smoking and the development of RA in the general population of Catalonia, Spain.
MethodsWe conducted a case–control study including all patients with a new diagnosis of RA registered in the SIDIAP database between 01/01/2008 and 31/12/2018; and matched them to up to 1:5 controls by age, gender and general practitioner. Smoking was classified by primary care staff into never, ex- or current smoking. Odds Ratios and 95% confidence intervals for the association between current and ex-smoking (compared to never smoking) and RA were estimated using conditional logistic regression adjusted for potential confounders.
ResultsA total of 13,920 RA cases and 69,535 controls were included. Compared with never smokers, current and ex-smokers were at increased risk of RA, with adjusted OR of 1.28 [95% CI 1.20–1.37] and OR 1.19 [1.12–1.26] respectively.
ConclusionOur findings confirm an association between smoking and the risk of developing RA. The effect seems to prevail in the long-term and even in ex-smokers for 2 or more years after smoking cessation. More research is needed on the effects of smoking discontinuation on RA prevention and related outcomes.
El tabaquismo es uno de los pocos factores de riesgo modificables asociados al desarrollo de artritis reumatoide (AR). La mayoría de los datos publicados tiene más de 10 años, y ninguno de ellos ha incluido a las poblaciones mediterráneas. Por ello, aprovechamos los datos recabados diariamente de manera rutinaria en atención primaria, para estudiar la asociación entre el tabaquismo y desarrollo de AR en la población general de Cataluña, España.
MétodosRealizamos un estudio de casos y controles, incluyendo a todos los pacientes con diagnóstico reciente de AR, registrados en la base de datos SIDIAP entre el 1 de enero de 2008 y el 31 de diciembre de 2018, y los pareamos con hasta 1:5 controles por edad, sexo y médico general. El tabaquismo fue clasificado por el personal de atención primaria como: no fumador, ex fumador, o fumador actual. Se calcularon los odds ratios y los intervalos de confianza del 95% para la asociación entre fumador actual y ex fumador (en comparación con no fumador) y AR, utilizando una regresión logística condicional ajustada a los factores potenciales de confusión.
ResultadosSe incluyó un total de 13.920 casos de AR y 69.535 controles. En comparación con los no fumadores, los fumadores actuales y los ex fumadores tuvieron un riesgo incrementado de AR, con un OR ajustado de 1,28 [95% IC de 1,20 a 1,37] y OR 1,19 [de 1,12 a 1,26], respectivamente.
ConclusiónNuestros hallazgos confirman la asociación entre tabaquismo y riesgo de desarrollo de AR. El efecto parece prevalecer a largo plazo, incluso en ex fumadores durante 2 o más años, tras haber dejado de fumar. Son necesarios más estudios sobre los efectos que tiene el dejar de fumar en la prevención de AR y los resultados conexos.
Rheumatoid arthritis (RA) is a long-term autoimmune disease which is thought to be related with genetic and environmental risk factors. One of the few modifiable risk factors is smoking. The relationship between smoking and RA has been shown in a lot of studies and reviews previously. The first study that related smoking with an increased risk of developing RA was published in 1987,1 and since then, numerous additional studies have been reported. Most of these studies were completed 10 or more years ago, and both the diagnosis and treatment/s available for RA have changed substantially in the last decade. Additionally, the routine collection of health data for research has now enabled the conduction of bigger studies in actual practice conditions and using the entire population, in contrast with previous cohorts or case–control studies mostly conducted in specialised research centres.2 Finally, none of the studies published to date were conducted in Mediterranean populations, where both the incidence/prevalence and the severity of RA are lower than in Northern European and American people.3,4
We therefore took advantage of the existence of routinely collected health data from electronic primary care records in Catalonia (Spain) to study the association between smoking and the development of rheumatoid arthritis in the general population.
MethodsData sourceData were extracted from the SIDIAP (‘Sistema d’Informació per al Desenvolupament de la Investigació en AtencióPrimària’, English translation: Information System for Research in Primary Care) database. SIDIAP contains primary care electronic medical records covering over 80% of the Catalan population (approximately 6 million people's records). These records include socio-demographics, lifestyle risk factors and measurements (body mass index, smoking, alcohol consumption), and diagnoses coded using the ICD10 system, amongst other variables.
Study design and participantsWe conducted a population-based case–control study including all participants newly diagnosed with rheumatoid arthritis registered in SIDIAP in the period 2008–2018 and with at least 2 years of data available prior to RA diagnosis. Up to 5 RA-free controls registered in any of the practices contributing to SIDIAP and with a similar run-in period (2+ years) were identified and matched by age, gender and general practitioner to each RA case. Controls were given the same index date as their matched case.
OutcomeThe outcome of interest of this study was the diagnosis of RA, defined by a previously validated list of ICD10 codes, including M05, M06 and all sub-codes. Previous validations of RA in SIDIAP5 have shown face validity, with population-based incidence and prevalence similar to that previously reported for the source population. In addition, a total of 2482/5796 incident cases studied in the validation manuscript had evidence of rheumatoid factor testing in primary care, with a 73.9% being positive, in line with previous knowledge.
ExposureSmoking is routinely recorded by family physicians in the Catalan healthcare system as part of their day-to-day job. Smoking status is recorded in a structured format into three categories: never smoker, ex-smoker, or current smoker. For the current study, we classified participants based on the most recent record in the two years prior to their index date.
Statistical analysesBaseline characteristics for study participants are reported as mean (standard deviation) or n (%) stratified by RA status (cases vs controls). Conditional logistic regression models were used to calculate unadjusted (sex- and age-matched) Odds Ratios and 95% confidence intervals for the association between current and ex-smoking and RA status. In addition, multivariable conditional logistic models were then used to adjust for potential confounders, including body mass index, socio-economic deprivation, alcohol drinking, cardiovascular/cerebrovascular disease, history of osteoarthritis, peripheral artery disease, chronic kidney disease, diabetes mellitus, cancer, inflammatory bowel disease, and number of GP visits in the year before index date. Gender-stratified analyses were conducted as there were significant differences in effect size seen in previous literature.6,7 Multiple imputation with chained equations (MICE) was used to minimise the impact of missing data for smoking, alcohol drinking, and body mass index. Ten datasets were imputed using MICE, and the results were combined using Rubin's rules. All analyses were conducted using Stata for Windows version 15.0.
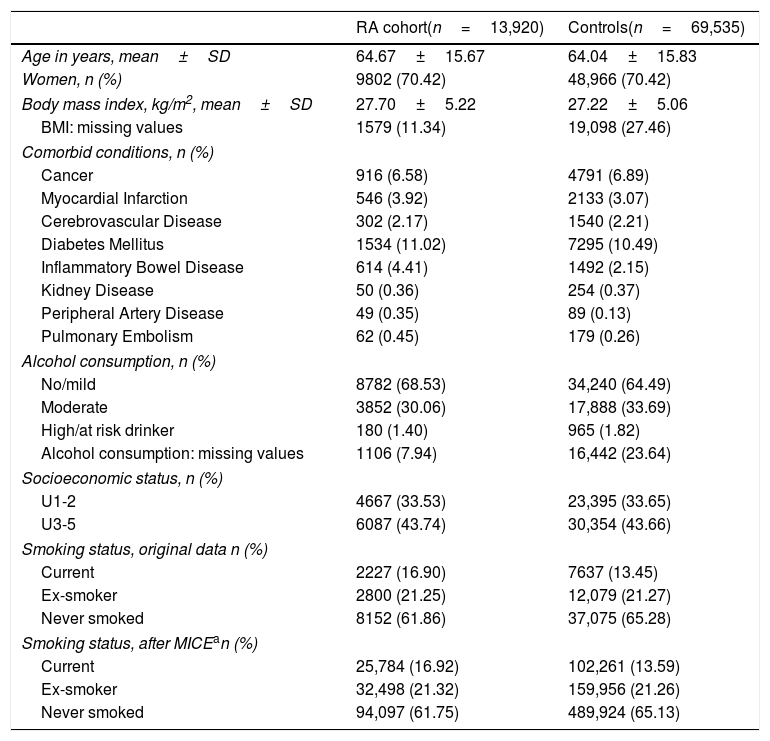
ResultsIn total, we included 13,920 RA cases matched to 69,535 controls with a similar age, gender and same primary care practice. Average (standard deviation) age was therefore similar between cases (64.67±15.67 years) and controls (64.04±15.83), and the proportion of females was the same in both groups (70.42%). In addition, body mass index was also similar between cases and controls, and the prevalence of co-morbidity was also comparable, except for inflammatory bowel disease, peripheral artery disease and pulmonary embolism, all three being more common amongst RA cases. Detailed baseline characteristics for cases and controls are reported in Table 1.
Baseline characteristics for cases and controls, including smoking status before and after multiple imputation.
| RA cohort(n=13,920) | Controls(n=69,535) | |
|---|---|---|
| Age in years, mean±SD | 64.67±15.67 | 64.04±15.83 |
| Women, n (%) | 9802 (70.42) | 48,966 (70.42) |
| Body mass index, kg/m2, mean±SD | 27.70±5.22 | 27.22±5.06 |
| BMI: missing values | 1579 (11.34) | 19,098 (27.46) |
| Comorbid conditions, n (%) | ||
| Cancer | 916 (6.58) | 4791 (6.89) |
| Myocardial Infarction | 546 (3.92) | 2133 (3.07) |
| Cerebrovascular Disease | 302 (2.17) | 1540 (2.21) |
| Diabetes Mellitus | 1534 (11.02) | 7295 (10.49) |
| Inflammatory Bowel Disease | 614 (4.41) | 1492 (2.15) |
| Kidney Disease | 50 (0.36) | 254 (0.37) |
| Peripheral Artery Disease | 49 (0.35) | 89 (0.13) |
| Pulmonary Embolism | 62 (0.45) | 179 (0.26) |
| Alcohol consumption, n (%) | ||
| No/mild | 8782 (68.53) | 34,240 (64.49) |
| Moderate | 3852 (30.06) | 17,888 (33.69) |
| High/at risk drinker | 180 (1.40) | 965 (1.82) |
| Alcohol consumption: missing values | 1106 (7.94) | 16,442 (23.64) |
| Socioeconomic status, n (%) | ||
| U1-2 | 4667 (33.53) | 23,395 (33.65) |
| U3-5 | 6087 (43.74) | 30,354 (43.66) |
| Smoking status, original data n (%) | ||
| Current | 2227 (16.90) | 7637 (13.45) |
| Ex-smoker | 2800 (21.25) | 12,079 (21.27) |
| Never smoked | 8152 (61.86) | 37,075 (65.28) |
| Smoking status, after MICEan (%) | ||
| Current | 25,784 (16.92) | 102,261 (13.59) |
| Ex-smoker | 32,498 (21.32) | 159,956 (21.26) |
| Never smoked | 94,097 (61.75) | 489,924 (65.13) |
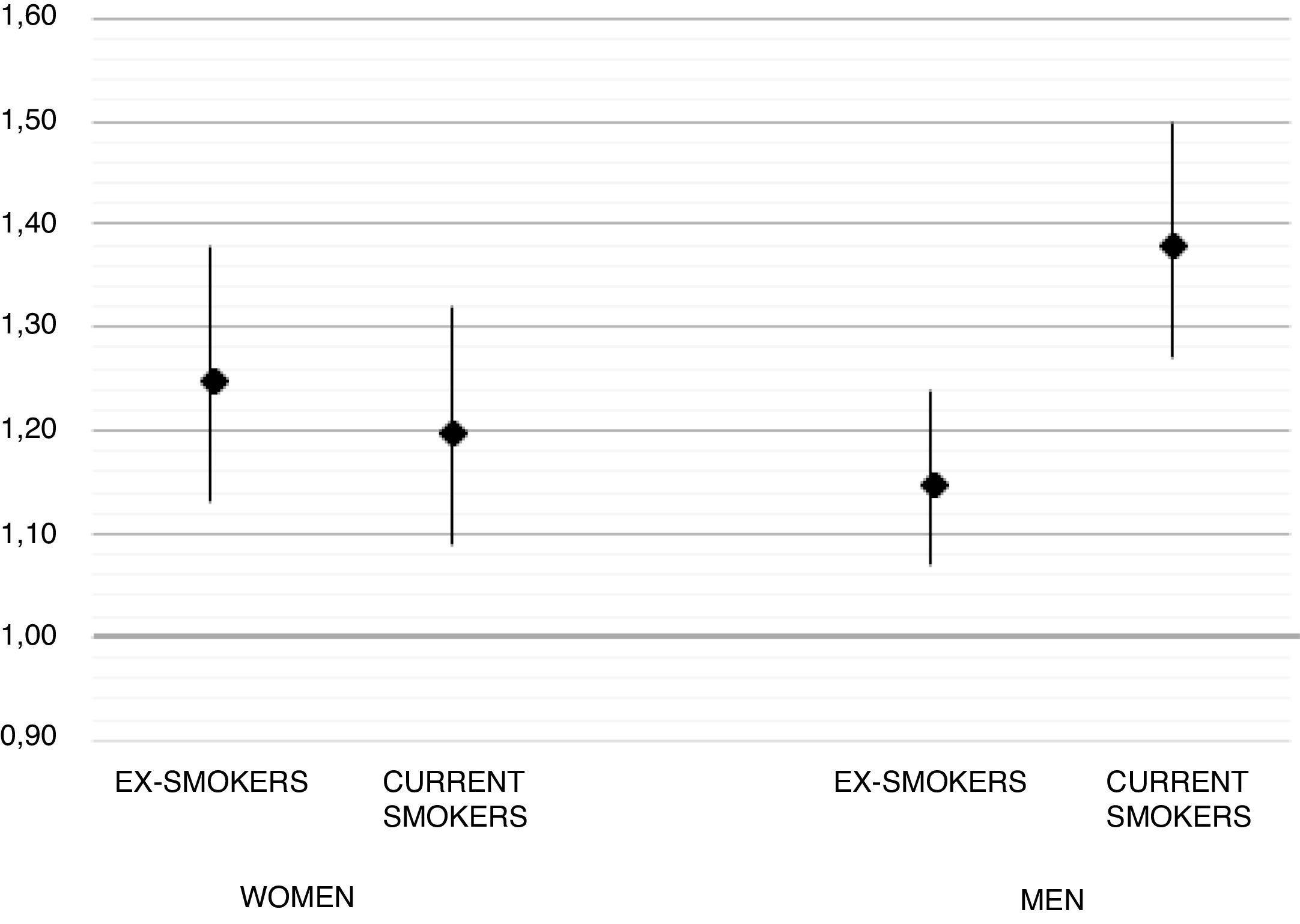
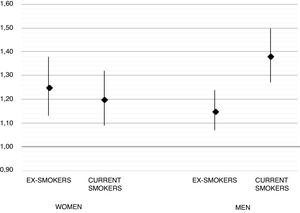
Before imputation, a total of 2227/13,920 (16.9%) of RA cases were current smokers, compared to 7637/69,535 (13.45%) controls, and remained similar after imputation (see Table 1). Unadjusted (sex, age, and GP-matched) OR for RA in ex-smokers was 1.09 [95% CI 1.03–1.14] and 1.35 [1.28–1.43] for current smokers. Multivariable adjusted ORs were 1.19 [1.12–1.26] and 1.28 [1.20–1.37] respectively. In the pre-specified gender stratified analysis, the observed association was stronger in men, with an adjusted OR 1.38 [1.27–1.50] for current smokers, compared to women (adjusted OR 1.20 [1.09–1.32] for current smoking). Full results for these models are depicted in Fig. 1.
DiscussionTo our knowledge, this is the largest and most up-to-date epidemiological study on the association between smoking and the development of RA to date. Our analysis is also the first to include a Mediterranean population, with previous studies conducted in Northern European,7 American,6 and Asian patients.8 Our study supports previous findings of an association between smoking and the risk of developing RA, with an almost 30% higher risk for current smokers and a 20% increase for ex-smokers compared to never smokers. In addition, we found a stronger effect of smoking amongst men, with an almost 40% excess risk in current smokers and a 15% in ex-smokers.
Our data do not differ from the results obtained in other studies done previously in different populations: most previous case–control studies found similar associations between current smoking and the risk of developing RA, with ORs ranging from 1.42 [1.13–1.80] to 2.37 [1.56–3.60].6,9 Similarly, cohort studies have mostly found an excess risk associated with smoking.10–12 Some controversial findings have however been published previously, that found no association between RA and smoking.13
Our study suggests that the effect of smoking on RA risk is long-lasting for years after smoking cessation, with ex-smokers still at an increased risk of developing RA compared to non-smokers. Interestingly, a recent study supports these findings: in a report by Seror et al.,14 passive smoking during childhood on the risk of developing RA.
Finally, other previous studies have shown3 like ours a stronger effect of smoking amongst men compared to women. It has been speculated that the observed difference in gender may be due to the difference in smoking intensity.3
One of the limitations of our study is the lack of detail on exposure intensity, i.e. cigarettes smoked per day or pack-years. Previous studies have reported on this, and found that smoking more than 20 pack-years make increase the possibilities of having RA.3 Also, with our data it is impossible to assess the timing when a person stopped smoking, and this makes ex-smoking effects difficult to interpret. An additional limitation is the use of routinely collected data, which was not designed for research purposes. Primary care coding of RA is likely delayed and/or focussed on more severe cases, as shown by the average age at diagnosis in our series, which is older than a recent study in Catalan population.15
On the other hand, our study has several strengths. This study includes the largest number of RA participants to date on this topic, with previous reports ranging from 90 to 7697 RA cases.12,16 Additionally, our study is the first one population-based, including all diagnosed cases of RA in a large population of around 6 million people, and in a Mediterranean region not previously studied. The diagnosis of RA has been previously validated in SIDIAP using laboratory and external rate comparisons.5 Finally, our controls were sampled from a universal primary care database making them representative of the general population. Matching on general practitioner minimised differences in socio-economic status, environmental factors, and physician differences in diagnostic or coding practices.
We confirm an association between smoking and the risk of developing RA. Research is needed on the impact of smoking cessation on the risk of developing this disease, and on related health outcomes including cardio-vascular disease and all-cause mortality in RA populations.
FundingThe research was partially supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). DPA is funded through an NIHR Senior Research Fellowship (Grant number SRF-2018-11-ST2-004). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health. APU is supported by Fundacion Alfonso Martin Escudero and the Medical Research Council (grant numbers MR/K501256/1, MR/N013468/1).
Conflict of interestDr. Prieto-Alhambra reports grants and other from AMGEN; grants, non-financial support and other from UCB Biopharma; grants from Les Laboratoires Servier, outside the submitted work; and Janssen, on behalf of IMI-funded EHDEN and EMIF consortiums, and Synapse Management Partners have supported training programmes organised by DPA's department and open for external participants. Mr Prats-Uribe reports grants from Fundación Alfonso Martin Escudero and the Medical Research Council. No other relationships or activities that could appear to have influenced the submitted work.