T-score bone mineral density (BMD) thresholds may influence guidance for treatment in patients under glucocorticoid (GC) therapy. Different BMD thresholds have been described but there is no international consensus. The aim of this study was to find a threshold to help in treatment decision-making in the population under GC therapy.
MethodsA working group representing three scientific societies from Argentina was convened. The first team was formed by specialists with expertise in glucocorticoid-induced osteoporosis (GIO) who voted according to summary of evidence. The second team was constituted by a methodology group who coordinated and supervised each stage. We conducted two systematic reviews to synthesize the evidence. The first included trials of drugs used in GIO to analyze the BMD cut-off used as inclusion criteria. In the second, we analyzed the evidence regarding the densitometric thresholds to discriminate between fractured and non-fractured patients under GC treatment.
ResultsIn the first review, 31 articles were included for qualitative synthesis and more than 90% of the trials included patients regardless of their densitometric T-score or range of osteopenia. In the second review, 4 articles were included and more than 80% of the T-scores were in the range −1.6 to −2.0. The summary of findings was analyzed and put to a vote.
ConclusionsWith more than 80% agreement of the voting expert panel, a T-score≤−1.7 was considered the most appropriate for treatment in postmenopausal women and men over 50 years of age under GC therapy. This study could help in treatment decision-making in patients under GC therapy without fractures but other fracture risk factors should certainly be considered.
Los umbrales del T-score de densidad mineral ósea (DMO) podrían influir en el tratamiento de pacientes bajo terapia con glucocorticoides (GC). Se han descrito diferentes umbrales, pero no existe un consenso internacional. El objetivo de este trabajo fue encontrar un umbral que ayude en la decisión terapéutica en la población bajo tratamiento con GC.
MétodosSe convocó un grupo de trabajo en representación de tres sociedades científicas de Argentina. El primer equipo estuvo formado por especialistas con experiencia en osteoporosis inducida por glucocorticoides (OIG), quienes estuvieron a cargo de la votación basada en la evidencia. El segundo equipo estuvo a cargo de la metodología coordinando y supervisando cada etapa. Realizamos dos revisiones sistemáticas: la primera incluyó ensayos de fármacos utilizados en OIG para analizar el T-score considerado como criterio de inclusión. En la segunda, analizamos la evidencia sobre umbrales densitométricos para la discriminación de pacientes fracturados y no fracturados bajo tratamiento con GC.
ResultadosEn la primera revisión se incluyeron 31 artículos donde se halló que más de 90% de los ensayos incluyeron pacientes independientemente del T-score o en el rango de osteopenia. En la segunda revisión se incluyeron cuatro artículos donde observamos que más de 80% de los valores de T-score se encontraban entre -1,6 y -2,0.
ConclusionesCon un acuerdo superior a 80% del panel de expertos, un T-score ≤ -1,7 se consideró el más adecuado para el tratamiento en mujeres posmenopáusicas y hombres mayores de 50 años bajo tratamiento con GC. Este estudio podría ayudar en la decisión terapéutica en pacientes bajo tratamiento con GC sin fracturas, pero ciertamente deberían considerarse otros factores de riesgos de fracturas complementarios.
Bone mineral density (BMD) measured by dual X-ray absorptiometry (DXA) is the current gold standard reference for the diagnosis of osteoporosis. Since BMD is one of the strongest predictors of fracture risk, many agencies worldwide have adopted BMD-based criteria as intervention thresholds.1 According to existing guidelines for postmenopausal women, a T-score of −2.5 SD or lower is recommended to offer treatment in those patients without fragility fractures.2 The T-score BMD thresholds might influence current guidance for treatment in patients under glucocorticoid (GC) therapy. Different BMD thresholds were described throughout the time.3–9 Several differences exist between the 2001 and 2010 American College of Rheumatology (ACR) glucocorticoid-induced osteoporosis (GIO) guidelines. In 2001, the ACR guideline recommended treatment for GIO in any patient with a T-score below −1.0. On the other hand, in 2010 the treatment was suggested considering the low, moderate, or high-risk of fracture. The ASBMR-PPC (American Society for Bone and Mineral Research-Professional Practice Committee) also stratified postmenopausal women and men≥50 years into low, medium, and high risk of fracture according to FRAX score. For patients who had low risk but have an indication for prednisone >7.5mg/day, the recommendation is to indicate treatment but for those with lower GC doses, the suggestion is to monitor the patient with BMD.6 In 2012, the IOF-ECTS GIO Guidelines Working Group defined a BMD T-score lower than −1.5 as one of the indications for bone-protective therapy in postmenopausal women and men ≥50 years under GC therapy.7 The Japanese guidelines also consider it as a cut-off value of BMD in around 80% of the young adult population.10 The ACR guideline 2017 recommended initial pharmacologic treatment in adults≥40 years of age who are at moderate-to-high risk of fracture considering high-risk to those with T-score≤−2.5, by FRAX GC-adjusted or with a prior fragility fracture. Recently, the 2020 GIO Brazilian guidelines recommended a T-score≤−1.89 for treatment in men.8 Consequently, there is no international consensus regarding the BMD cut-off for diagnosis and treatment in GIO.
Therefore, in the context of the development of Argentine guidelines for prevention and treatment of GIO, a working group on behalf of three scientific societies from Argentina: AAOMM (Asociación Argentina de Osteología y Metabolismo Mineral – Argentine Association of Osteology and Mineral Metabolism), SAO (Sociedad Argentina de Osteoporosis – Argentine Osteoporosis Society) and SAR (Sociedad Argentina de Reumatología – Argentine Society of Rheumatology) was convened to identify areas of consensus among a panel of experts, with the aim of discussing the available data for the threshold based on BMD for therapeutic decision-making in postmenopausal and men≥50 years under GC therapy. In the absence of a consensus about a cut-off for the diagnosis of GIO, there is a risk of not identifying those patients who, under GC therapy, are at risk of fragility fracture.
ObjectiveThe overall aim of this study was to find a threshold according to the reported evidence to be able to help in the treatment decision in the population under GC therapy.
MethodsDevelopment teamsThis work involved a working group on behalf of three scientific societies from Argentina: AAOMM, SAO and SAR. An equal number of participants was selected by each society according to its expertise.
The first team was formed by specialists (rheumatologists, endocrinologists, internists, physiatrists) with expertise and clinical experience in treating GIO and bone diseases. The second team was constituted by a methodology group who coordinated and supervised each stage of the work and conducted the literature search and data abstraction, rated the quality of evidence, analyzed the data, and created the tables of summary of findings. In the framework of two virtual meetings, the methodology and summary of findings of the two systematic reviews were shown to the voting expert panel who discuss about the threshold based on BMD for therapeutic decision-making in postmenopausal and men≥50 years under GC therapy. The voting expert panel voted both diagnostic questions requiring 70% agreement among the voting members. Diagnostic questions: 1. What was the densitometric threshold used as inclusion criteria in the population treated with GC in the trials that studied drugs for GIO? 2. What would be the best densitometric threshold that discriminates among fractures and non fractures in patient under GC treatment?
Systematic reviewsTwo systematic reviews were performed (Appendix 1). In the first systematic review the literature was searched for key terms until October 2020, using the databases of MEDLINE (National Library of Medicine) (https://pubmed.ncbi.nlm.nih.gov/), Cochrane Library (https://www.cochranelibrary.com/) and LILACS (https://lilacs.bvsalud.org/es/), and grey literature in the last 5 years. This review was performed in the context of the development of Argentine guidelines for prevention and treatment of GIO under PICO structure. In this first review, the focus was to evaluate the T-score cut-off used in the trials that evaluated pharmacological intervention in GIO. In the second systematic review, the literature was searched for key terms until February 2022 and aimed to search for evidence about the BMD T-score threshold that discriminates between fractured and non-fractured populations. An expert panel reviewed the included studies selected in both systematic reviews.
Eligibility criteriaWe included randomized controlled trials, non-randomized trials, post hoc analysis and pooled analysis in postmenopausal women and men older than 50 years old, in which the T-score BMD baseline was reported studying a pharmacological treatment in patients under GC therapy (≥5mg/day prednisolone or equivalent) regardless of GC administration time. T-scores BMD in the lumbar spine (LS) or total hip (TH), thresholds T-scores BMD values were considered as outcomes. Only articles in English, Portuguese and Spanish were included.
Case reports, reviews, letters to the editor, animal studies, editorials, commentaries, other languages than the described previously, and if identical data were re-analyzed were considered as exclusion criteria.
Study selectionRayyan software (https://rayyan-prod.qcri.org/welcome) was used to screen the literature search results. Two independent reviewers performed the title and abstract screening and then underwent full-text screening. A third reviewer was included in case of conflicts.
The data extraction and processing were obtained by two authors independently using a standardized data extraction: authors, study design, participants, age of participants, time and dose of GC, diagnostic used for osteoporosis, baseline T-score value in each group, intervention, comparator.
The quality assessment and publication bias of randomized controlled trials were judged using the Cochrane risk of bias tool (http://handbook.cochrane.org/). For non-randomized controlled trials, the assessment was performed using the Newcastle–Ottawa scale (NOS).11 For cross-sectional studies, we used a modified version of NOS and studies that received at least seven stars (maximum of ten) were classified as good quality. The PRISMA-P guideline was used to prepare this systematic review.12,13
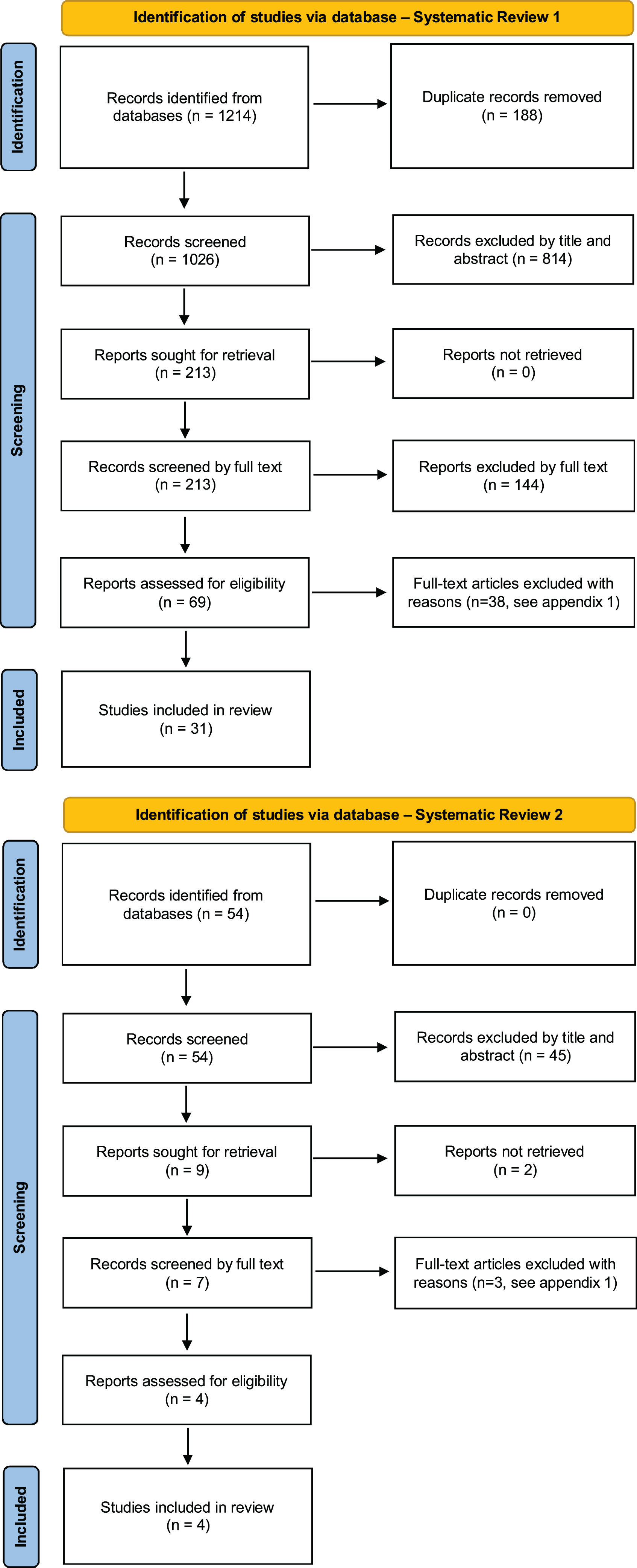
ResultsQualitative synthesis. Description of studiesThe studies were divided into two parts according to each search: Part 1 included the trials of drugs used in GIO, while Part 2 included BMD thresholds for fractured and non-fractured patients’ discrimination under GC therapy. The flowcharts of both systematic reviews are shown in Fig. 1, and the quality assessment of the studies is in Appendix 2.
Part 1In this first revision, a total of 1214 selected articles were found. A total of 31 articles were included for qualitative synthesis and we stratify the trials according to the densitometric T-score used as inclusion criteria in each trial.14–44
The summary of the studies characteristics is shown in Appendix 3. The 93.7% of the trials included patients regardless of the densitometric T-score or in the range of osteopenia (Table 1).
T-score as densitometric inclusion criteria in trials about GIO.
| Densitometric inclusion criteria | n | % |
|---|---|---|
| Regardless of their baseline BMD. | 1714,16–19,21–25,28–30,34–36 | 53.1 |
| BMD T-score at the lumbar spine (LS) and total hip (TH): either ≤−2.0, or ≤−1.0 in addition to at least one fragility fracture during treatment with GC. | 538–42 | 15.6 |
| GIO according to Japanese Society for Bone and Mineral Research criteria (2004): T-score −1.7; 80% YAM. | 315,26,37 | 9.4 |
| BMD T-score at the LS or TH: ≤−2.5. | 420,27,33,43 | 12.5 |
| BMD T-score at the LS: ≥−2.0. | 131 | 3.1 |
| BMD T-score at the LS: <−1.0 and ≥−2.5. | 132 | 3.1 |
| BMD T-score at the LS or TH: <−1.0. | 144 | 3.1 |
Abbreviations: BMD: bone mineral density; GIO: glucocorticoid-induced osteoporosis; LS: lumbar spine; TH: total hip; YAM: young adult mean.
In this second revision a total of 54 selected articles were found and 4 articles were included.45–48 More than 80% of the T-scores were between the −1.6 and −2.0 range (Table 2).
Analysis of the BMD T-score threshold for fractured and non-fractured discrimination in patients under GC therapy.
| Description | Population/site | T-score |
|---|---|---|
| van Staa, et al.45 analyzed the BMD threshold for vertebral fracture in postmenopausal women taking GC versus placebo from 2 randomized clinical trials (prevention and treatment trials of risedronate).Total: 111 postmenopausal women (risedronate group), 56 (placebo group).Age: 59.6±1.0 (risedronate group), 57.1±1.3 (placebo group).Underlying diseases: rheumatic, pulmonary and skin disorders. | Control patients | |
| Lumbar spine | −2.6 | |
| Femoral neck | −2.6 | |
| GC treated patients | ||
| Lumbar spine | −1.8 | |
| Femoral neck | −1.9 | |
| Nawata H, et al.46 studied the BMD T-score cut-off which discriminates among fractured and non-fractured patients.Total: 692 patients (627 women and 65 men).Underlying diseases: rheumatoid arthritis (RA, n=319), systemic lupus erythematosus (SLE, n=162), progressive systemic sclerosis (n=27), mixed connective tissue disease (n=26), polymyositis/dermatomyositis (n=20), polymyalgia rheumatica (n=16), nephropaty (n=12), and 110 with other diseases. | In all population | |
| Primary osteoporosis | −2.60 | |
| Osteopenia | −1.70 | |
| GC treated patients: All population | −1.97 | |
| GC treated patients: RA population | −2.24 | |
| GC treated patients: SLE population | −1.60 | |
| GC treated patients: Non-RA population | −1.40 | |
| According to GC doses | ||
| ≥5mg/day | −1.90 | |
| ≥7.5mg/day | −1.67 | |
| ≥10mg/day | −1.52 | |
| RA population (≥7.5mg/day) | −2.12 | |
| Kumagai S, et al.47 is q cross-sectional study in women who had received at least 0.5mg/kg of oral GC for more than 1 month.Total: 160 Japanese womenAge: 16–85 yearsUnderlying disease: autoimmune diseases. | Premenopausal women | −1.7 |
| Postmenopausal women | −2.1 | |
| Kaji et al.48 studied the relationship between the presence or absence of vertebral fractures and BMD in 136 females Japanese patients treated with GC. They analyzed the cut-off values on BMD for the incidence of vertebral fractures in those patients compared with controls.Underlying disease: autoimmune disease (n=102), neurological disease (n=15), dermatological disease (n=6), respiratory disease (n=5), inflammatory bowel disease (n=5), haematological disease (n=5). | Control patients | |
| L2–L4 | −2.36 | |
| Femoral neck | −1.74 | |
| Radius (1/3) | −2.12 | |
| GC treated patients | ||
| L2–L4 | −1.88 | |
| Femoral neck | −1.67 | |
| Radius (1/3) | −1.55 | |
Abbreviations: BMD: bone mineral density; GIO: glucocorticoid-induced osteoporosis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus.
After analyzing the evidence from both systematic reviews, the expert panel discussed in meetings in the framework of a working group with experts in bone diseases. The expert panel voted on the threshold of the BMD T-score that best identifies fractured from the non-fractured population. Taking into account that more than 90% of the trials about GIO included patients regardless of the densitometric T-score or in the range of osteopenia, and more than 80% of the T-scores that discriminates between fractured and non-fractured patients are within −1.6 and −2.0, the voting was performed among this range.
With an agreement over 80% of the voting expert panel, a T-score≤−1.7 was considered the most appropriate for treatment in postmenopausal women and men older than 50 years under GC therapy.
DiscussionThe T-score BMD thresholds might influence current guidance for treatment in patients under GC treatment. Several published guidelines for GIO management demonstrate large differences in the thresholds in patients under GC treatment as were previously detailed. In GIO there is no international consensus regarding the most appropriate BMD threshold for treatment. Therefore, we considered conducting this systematic review in the context of the development of Argentine guidelines for prevention and treatment of glucocorticoid-induced osteoporosis by 3 scientific societies from Argentina to determine the most appropriate BMD T-score threshold to help in the GIO treatment decision.
As far as we know, the thresholds are noy ideal from every perspective and there is no T-score BMD threshold gold standard established by international consensus in GIO. At the same time, the fracture incidence differs markedly in different regions of the world, accordingly to ethnicity among other variables.49 Despite it being suggested that each country should determine their own intervention thresholds, based on fracture incidence, availability of resources, and economic considerations, these data are not always available. Therefore, the fracture risk assessment tool (FRAX) was developed to estimate the 10-year probability of fractures.50 Dawson-Hughes et al. identified cost-effective intervention thresholds based on 10-year absolute hip fracture risk. With BMD in osteopenia levels (T-score −2.0) plus a clinical risk factor, the absolute fracture probability estimate meets or exceeds the 3% cost-effectiveness threshold in all instances.51
Previous studies have evaluated BMD thresholds for discrimination of fractured and non-fractured patients under GC therapy.45–48 Kaji et al. analyzed the threshold of BDM for vertebral fracture in Japanese female patients with GIO.48 The cut-off values of BMD which discriminates patients with vertebral fractures from those without vertebral fractures were higher in patients with GC treatment. They found a cut-off for vertebral fracture of −1.88 in lumbar spine BMD (sensitivity and specificity 61.5%) and −1.67 in femoral neck BMD (sensitivity and specificity 70.9%). Nawata et al., in the context of the GIO Japanese guidelines, described the results of a longitudinal study in which the BMD T-score cut-off was discriminated between fractures and non-fractures.46 Considering the whole GC treated group (n=692), the cut-off found was a T-score of −1.97. Van Staa TP et al., after analyzing data from 2 large, prospective, randomized controlled trials (n=306) showed that the daily GC dose, and not the cumulative dose, is a strong predictor of the risk of vertebral fracture in postmenopausal women under GC therapy, at similar baseline levels of BMD. They described a T-score threshold of −1.8 for the lumbar spine and −1.9 for femoral neck BMD.45
In summary, the voting expert panel selected a BMD T-score≤−1.7 as the most appropriate for help in the treatment decision in postmenopausal women and men older than 50 years under GC therapy. This agreement was reached after prolonged discussion and debate. Lower T-scores were not selected because lack of evidence was considered and to avoid overtreatment even when there is not enough data on the different ethnic groups and populations.
One of the limitations of this paper is that the analysis is based on indirect data from systematic reviews and most data come from the Japanese population. However, two exhaustive systematic reviews were conducted, addressing the problem from two perspectives as a strength. Therefore, longitudinal studies according to age, gender and ethnicity are needed to identify the optimal BMD threshold.
ConclusionsWith an agreement over 80% of the voting expert panel, a T-score≤−1.7 was considered the most appropriate for treatment in postmenopausal women and men older than 50 years under GC therapy. This study could help in the treatment decision in patients under GC therapy without fractures but certainly should consider other concepts as age, sex, fracture history, risk fracture stratification, FRAX, among others.
Authors’ contributionMLB: study design and scope of these systematic reviews. MLB, MSL, LFS, EG, MD, AMG, LARS, MDLV: voting expert panel. MLB, AR, LARS, CM, LRB: performed the systematic review. MLB and LRB: drafting of the manuscript. All authors read, discussed, and approved the final manuscript.
Financial supportNone.
Conflict of interestMaria Lorena Brance received lecture fees from Amgen and Craveri and fees as an advisory board from Amgen. María Silvia Larroudé received lecture fees from Pfizer, Lilly, Amgen, Astrazeneca and Genzyme Sanofi. Evangelina Giacoia received lecture fees and fees as an advisory board from Amgen. María Diehl received research support from Amgen. Ana María Galich received lecture fees from Raffo, Amgen and Eli Lilly. Luis Fernando Somma, Luis Agustín Ramirez Stieben, María Cielo Maher, Maria Celina de la Vega, Ariana Ringer, and Lucas R Brun reported no conflicts of interest.