Pregnancy is a situation of semi-allogenic immunological tolerance with regard to the fetus. The mechanisms of tolerance are described as uterine changes induced by the syncytiotrophoblast over: cytokines, T and natural killer (NK) lymphocytic subpopulations and complement, as well as the presence of human leukocyte antigen (HLA)-C class II, HLA-E and HLA-G. Moreover, systemic immunological changes are also produced: thymic involution through progesterone, decreased NK activity and a change toward an anti-inflammatory profile of cytokines (T helper cells [Th2]).1
Monoclonal antibodies (mAb) have revolutionized the treatment of autoimmune and inflammatory diseases, so frequent among women of childbearing age. Therefore, their utilization before or during pregnancy, is sometimes proposed, as a question of clinical practice. The mAb used, at the present time, are not apt for utilization during pregnancy2 and there are no controlled studies in pregnant women. According to the Unites States Food and Drug Administration (FDA), these mAb are classified as category B (infliximab), as are their biosimilars, adalimumab, etanercept, golimumab and certolizumab pegol [CZP]), and as C (rituximab, tocilizumab and abatacept). There have been no significant differences in the rate of abortions among patients exposed to infliximab and naïve patients.3,4
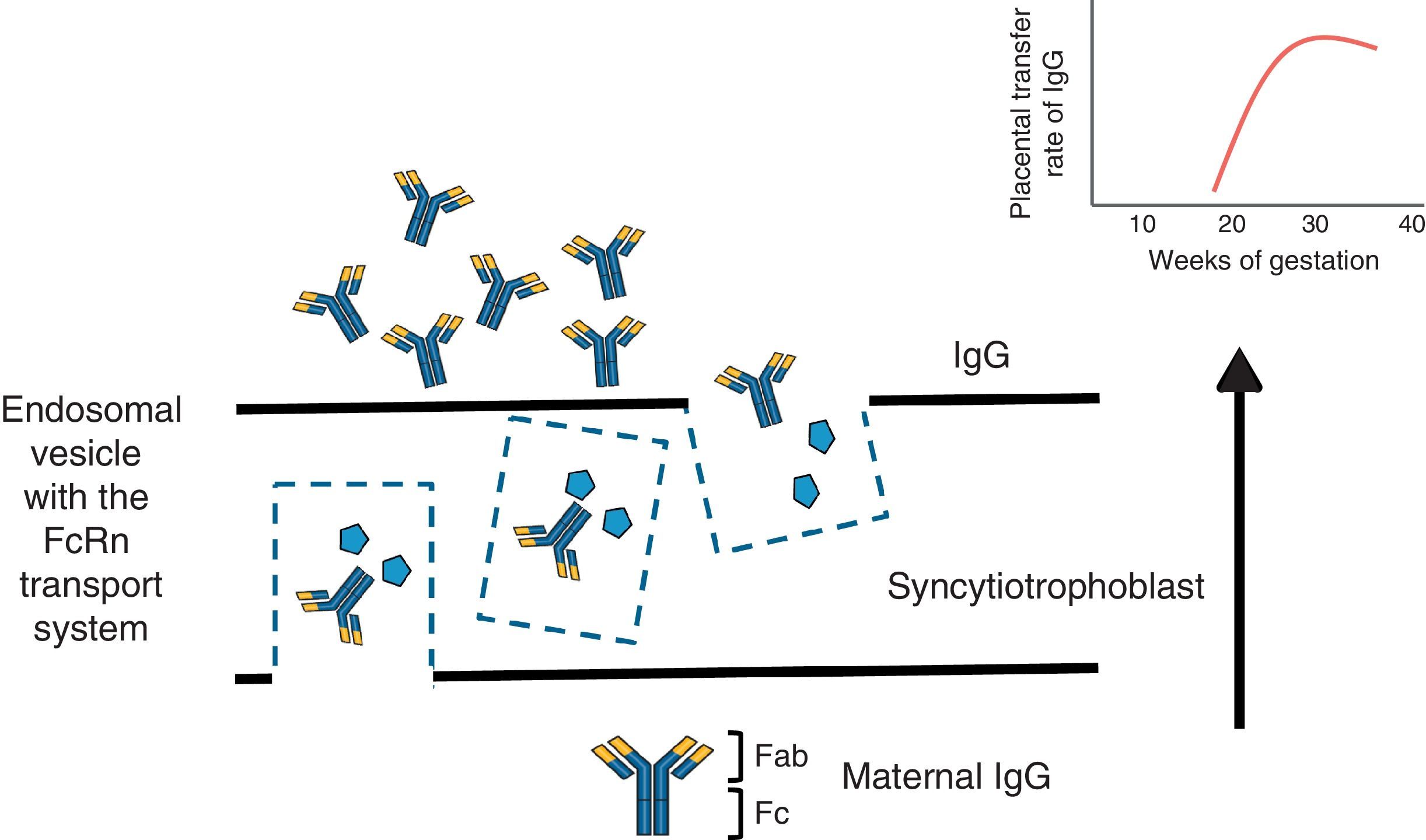
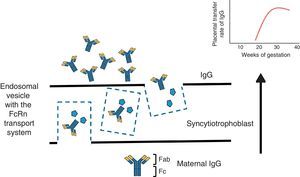
To understand the process of placental transfer of mAb would help us to evaluate the risk of beginning or maintaining their administration during pregnancy. Nutrients are transferred to the fetus through a maternal layer of syncytiotrophoblasts and another of endothelium (fetal capillaries) by simple diffusion or using transport proteins. Toxins are returned by the fetus to the maternal circulation to be eliminated. Composed of low molecular weight (<500Da), like O2 and amino acids, they diffuse passively through the placenta, but those with a high molecular weight require transport proteins to cross it. Immunoglobulin G (IgG) has a molecular weight of 160kDa and crosses the placenta through the neonatal Fc receptor (FcRn) present in the syncytiotrophoblast cells.5,6
The structure of most of the mAb utilized contains a constant region of IgG1 (Fc) and, during the first 20–22 weeks of pregnancy, there is a minimal active transfer because of the absence of FcRn. Transport across the placenta increases significantly toward the third trimester of the pregnancy (Fig. 1). Certolizumab pegol contains a PEGylated Fab fragment of the anti-tumor necrosis factor (TNF) antibody, and lacks the Fc fragment. Therefore, CZP crosses the placenta by passive diffusion more than by active transport utilizing FcRn, and, thus, the placental transfer of CZP must be minimal.7 Likewise, although it cannot be explained by the FcRn system, observations on the levels of etanercept in umbilical cord have demonstrated that the rate of placental transfer is very low.8 Mahadevan et al.,9 showed that concentrations of infliximab and adalimumab, but not CZP, are greater in umbilical cord than in maternal serum; mean levels of infliximab and adalimumab in cord reach concentrations between 150% and 160% greater than the concentrations in maternal serum.9
There are other circumstances in which placental transfer of antibodies after weeks 20–22 can be detrimental to the fetus/neonate. Typically, in cases of rheumatoid arthritis and systemic lupus erythematosus, the passage of anti-Ro/SSA and/or anti-La/SSB autoantibodies of IgG isotype can cause an eruption due to exposure to the sun or the development of congenital heart block, which affects roughly 2% of fetuses/neonates of patients who have these autoantibodies.6
With the lack of data on the safety of mAb, the performance of clinical trials in pregnant patients is ruled out for ethical considerations, and the decision to use them would depend in each case on the clinical situation, as well as that of the potential benefits and risks for the mother, fetus or newborn. Long-term observational studies would enable us to confirm the efficacy and safety of category B mAb during pregnancy, to determine whether gestational exposure to mAb involves a long-term risk for the immune system being developed in the newborn, or should this vary depending on the trimester for exposure. It is important to consider that the administration of vaccines with alive or attenuated virus or bacteria, an indication that is present in certain vaccine calendars for newborns, for example, with bacillus Calmette-Guérin (BCG), with which, infection can have a fatal outcome.10 For these reasons, it is recommendable that they be postponed until the sixth month of life.
Conflicts of InterestLV: Abbvie, Roche Farma, Bristol-Myers Squibb, Pfizer, UCB, MSD and GSK; JGOB: nothing to declare; DHF: nothing to declare; FJLL: Abbvie, Roche Farma, Bristol-Myers Squibb, Pfizer, UCB and MSD.
Please cite this article as: Valor L, Ovalles-Bonilla JG, Hernández-Flórez D, López-Longo FJ. Una situación especial: tratamiento con anticuerpos monoclonales y embarazo en mujeres con enfermedades inflamatorias sistémicas. Reumatol Clin. 2016;12:359–360.