Systemic lupus erythematosus is a heterogeneous rheumatic systemic disease with extremely varied clinical manifestations and a diverse pathogenesis, as illustrated in this review on the most relevant new knowledge related to the disease. Topics such as anemia, pathogenesis, cardiovascular risk assessment, antiphospholipid syndrome, prediction of damage and recent advances in treatment, including tolerogenic and biological agents, are discussed. Relevant contributions regarding classical therapies such as corticosteroid and antimalarials and their optimal use, as well as the roll of vitamin D, are also referred.
El lupus eritematoso sistémico es una enfermedad reumática sistémica enormemente heterogénea, con múltiples posibles manifestaciones de patogenia diversa, como se ilustra en esta revisión sobre las novedades más relevantes concernientes a esta compleja enfermedad autoinmune. Se revisan aspectos como la patogenia de la anemia crónica asociada al lupus eritematoso sistémico, la estimación del riesgo cardiovascular, el síndrome antifosfolipídico, la predicción del daño acumulado y los avances más recientes en el tratamiento, incluyendo los tolerógenos y las terapias biológicas. También se revisan las contribuciones más relevantes en torno a las terapias clásicas, como la optimización del uso de los glucocorticoides y los antipalúdicos, así como el papel que pueda desempeñar la vitamina D.
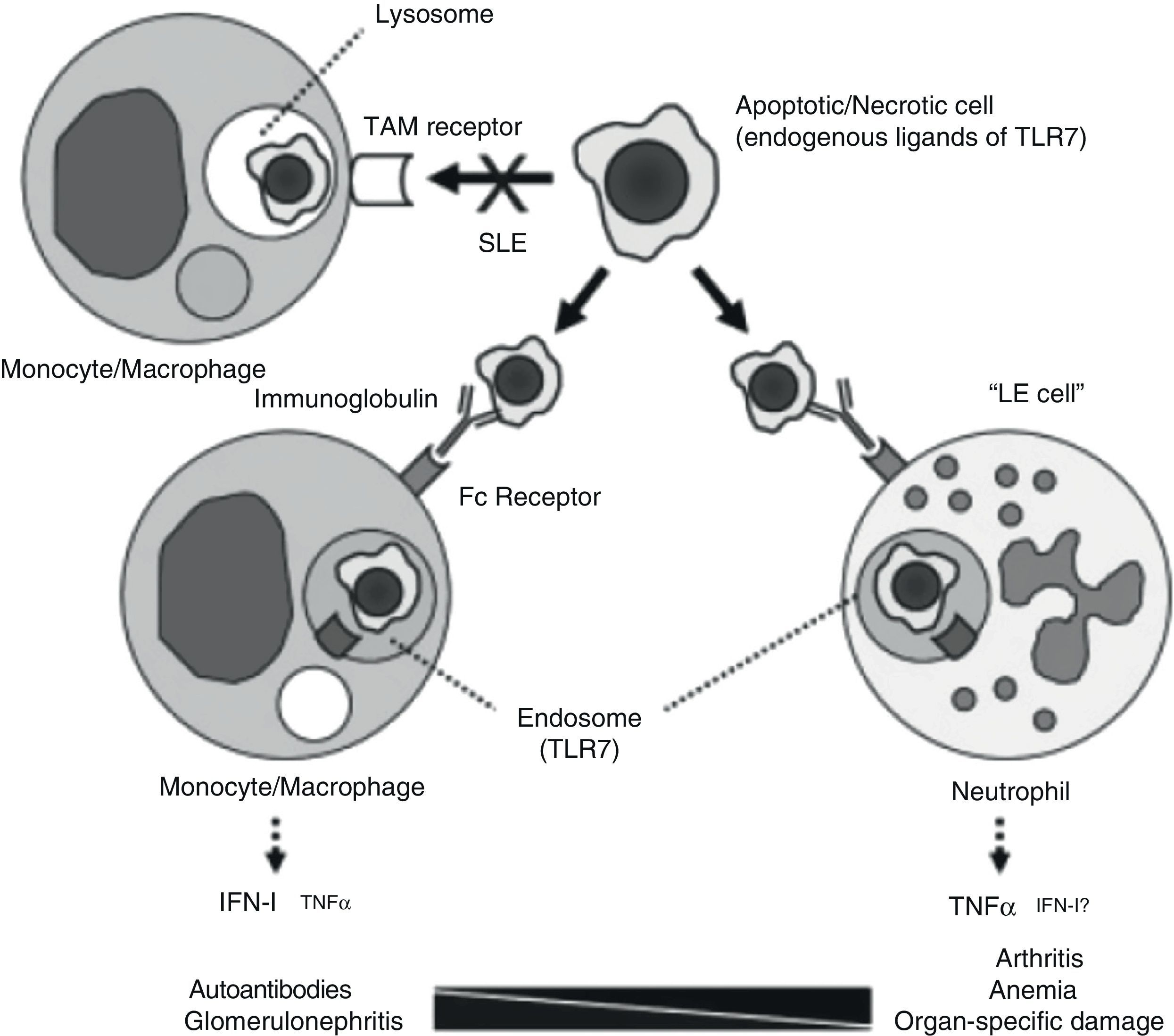
If there is something that characterizes systemic lupus erythematosus (SLE) it is diversity. Underlying such a wide array of clinical manifestations there are different pathogenic mechanisms. A study undertaken at the university of Florida, analyzing the pathogenesis of chronic anemia in SLE which, being the most frequent of anemia in SLE, it is the one with the most questions about it.1 The authors retrospectively review bone marrow biopsies of 6 patients with SLE and find, confirming most of the published, an important dyserythropoiesis, with abundant LE cells and marked apoptotic phenomena; they also prove, through immunohistochemical techniques, an increased presence of TNF and apoptosis biomarkers, as well as cleaved caspase 3. The authors test different hypothesis in knockout pristane-induced murine drug-induced lupus models. Among other findings, the abovementioned pathologic anomalies, which also developed in the mouse, did not require the intervention of the interferon alpha receptor and, however, did require a conserved TLR-7/TNF-¿ pathway. An excessive production of TNF on the part of neutrophils seems critical for the development of anemia and, possibly, of lupus related arthritis, as is proposed in their model (Fig. 1). This model questions the existing paradigm of type I INF mediation in all of the manifestations of SLE, carrying out a more relevant role in those in which the intervention of autoantibodies is critical, such as lupus glomerulonephritis (LN)). The possible therapeutic implications of the diversity of pathogenic mechanisms are obvious. It is necessary to point out that these patients were probably selected due to their severity and, therefore, there is the chance that they do not represent all of the lupus patients with non-hemolytic anemia.
Pathogenic model for different manifestations of SLE. The processing of apoptotic bodies would not be produced through TAM type receptors (Tyro3, Axl, Mer), but once opsonized by immunoglobulin, would be recognized by FC receptors on monocytes/macrophages or neutrophils, forming endosomes and relating to TLR 7, leading to the transcription of IFN or TNF, depending on the cell implicated.
It is clear that there is a problem with the personalized evaluation of the level of cardiovascular risk (CVR) in our patients with SLE. Patients with SLE have a greater CVR than age and gender matched population controls and this excess in relative risk is not explained only by an increase in the classical risk factors.2 Therefore, it is to be expected that the instruments based on the Framingham data, such as SCORE, are not useful in patients with SLE because they do not adequately capture the level of CVR of these patients. The group headed by Bevra Hahn has carried out a prospective observational study with the objective of identifying factors that predict the development of carotid atheroma plaque and/or its progression. They included a series of biomarkers with high pathogenic specificity implicated (according to previous studies) in the development of atheromatosis in SLE or the general population. For this analysis, they use a powerful computer tool with the objective of identifying predictors and the cutoff thresholds of biomarkers: the CART analysis or the classification and regression Trees.3 A series of explanatory variables were associated to the presence of plaque, with a high OR for leptin levels >34ng/dl (OR=7.3; 95% CI, 2.2–24.0; P=0.001), TNF-like weak apoptosis inductor (TWEAK) levels >373pg/ml (OR=28.8; 95% CI, 2.9–281.1; P=0.004) and proinflammatory HDL ≥0.94 FU (OR=9.1; 95% CI, 3.3–24.6; P<0.001). Diabetes mellitus reached a markedly high OR (OR=61.8; 95% CI, 6.4–598.1; P<0.001). The authors developed as score, which they labeled with the acronym “PREDICT”, whose predictive capacity for plaque was superior to the classical risk factors in positive predictive value (PPV) (63.5 vs 53.6), negative predictive value (NPV) (94.4 vs 74.7) and area under the curve (0.84 [0.78–0.90] vs 0.58 [0.49–0.67]).4 The study was not designed to predict events ad patients under treatment with statins were excluded, limiting the generalization of the results. In addition, the tests for the determination of several of these biomarkers are not widely available and lack enough standardization as to be applied generally. In any case, the main point of this study is that it is possible to improve the prediction of CVR in patients with SLE and that biomarkers, as opposed to what happens in the general population, will probably play a key role as prediction instruments.
A study by the SLICC group on their prospective cohort has found a large proportion of metabolic syndrome in patients with SLE (approximately 35%), which was associated to activity and damage, as well as age and African-American or Hispanic ethnicity. However, the real contribution of this finding regarding the estimation of the CVR is doubtful given that the group of factors defining metabolic syndrome has not been shown to be more than the sum of its components.5
Antiphospholipd SyndromeRecently, the 10 year results of the Europhospholipid Project, a multicentric European cohort constituted initially by 1000 patients with antiphospholipid syndrome (APS), have been published.6 The objective of this observational and prospective study was to analyze the morbidity and mortality of the syndrome, associated to SLE in 36.2% of cases. Only 40% of patients remained anticoagulated throughout the study and 135 did not receive any antithrombotic (platelet antiaggregant or anticoagulant), of which 93.3% were free of thrombosis at 10 years, suggesting that their physicians’ decisions were not off target. The predominance of venous thrombosis upon entry into the cohort stands to attention, with arterial thrombosis being however important by the end of the observation period. The researchers proposed, as an explanation to this finding, an improved efficacy on antithrombotic measures for the prevention of venous vs arterial thrombosis. An alternative explanation might be the difficulty in attribution, as an important percentage of patients have SLE and their predisposition to develop atherosclerosis is well known. In this sense, the paper does not report any effort to discriminate between thrombotic or atherosclerotic stroke. Anther limitation of the study is the lack of randomization upon inclusion, as well as the important percentage of patients lost to follow-up, which, excluding deaths, was of 41.9%. The standardized mortality ratio was 1.8 (95% CI, 1.5–2.1), a ratio similar to what has recently been reported in the European SLE cohorts.7 Among the most common causes of death, the first is thrombosis: 36.5% (3% of total). Maybe reflecting the already mentioned large representation of patients with SLE in the cohort, infection was also a frequent cause of death (26.9% of cases) and, standing out, 10.7% of the patients died as a consequence of hemorrhage. There is clearly a need for more studies, with a careful evaluation of the risk-benefit ratio that improves the basis of decisions concerning APS-related anticoagulation.
Damage PredictorsCumulative damage, mostly related to long-term use of glucocorticoid steroids (GC),8 is a major clinical problem in SLE, one still without a solution. Several cohorts have recently published prospective data looking for damage predictors and the SLICC group has done so in its large inception, multicentric and multinational cohort.9 The study included 1502 patients with a mean±standard deviation of 4.25±2.7 visits per patient. They found a series of modifiable factors associated to damage progression, measured by the group's own score (SDI), such as SLE disease activity (SLEDAI-2k), GC and hypertension, replicating the results of other studies. The authors made a special effort to try and explain the colinearity between GC and activity (that is, more activity, more GC), showing an independent effect of GC on damage. In essence, the relationship between activity and transition to damage (from non-damage) was greater in patients treated with steroids: 1.33 (95% CI, 1.02–1.74). Once again antimalarial appears as protective, with the common problem of their being confounding factors prescribed for milder cases of SLE. As had to happen, damage predicted mortality, with a hazard ratio of 1.46 per SDI point. The study also provides a rate of damage in an inception cohort, which will prove very useful for future research. A major weakness is that the effect of GC is poorly captured (cumulative dose was not calculated and the current dose was not mentioned), when the current dose of steroid is a strong predictor of damage.10
Toleragens to the Stage, Once AgainThe search for tolerance-inducing agents, in other words, agents capable of restoring tolerance to nuclear self-antigens, has been up until now as intense as useless in the case of SLE. Initially, promising drugs such as edratide or abetimus, with favorable serological effects have however failed to achieve clinical benefit. The recent appearance of lupuzor, also called regerimod, may revert this tendency. This drug, developed by the European biotechnology company ImmuPharma, is constituted by a small fragment of phosphorylated ribonucleoprotein in the 140 serine position, which seems to confer a T cell modulating capacity. With lupuzor there is an expansion of the regulatory phenotypes, reducing the effector elements, something that has been seen with other toleragen agents. Some unforeseen effects on the dendritic cell, such as the interaction with HSP-70 (or chaperones, highly expressed in autoimmune responses), may be important in its mechanism of action, destabilizing certain proteins, such as HLA class II proteins, with the resulting interference on antigen presentation. Whatever its mechanism, it is still unclear. Lupuzor has shown promising data in a phase IIb randomized controlled trial (RCT), added to standard treatment, with a high index of responders measured by the SLE responder index (SRI) at 24 weeks, up to 84.2%, if applied only to those patients with a clinical SLEDAI (without serological parameters) ≥6. In this double blind trial, a strong response to placebo was noted (over 40%), something that the authors attempted to explain by the release of favorable results upon snapshot analysis, carried out at 12 weeks.11 There were no important safety issues and no CD4 response to pathogens was seen. In spite of the groups not being well balanced after randomization and the primary objective was not reached, these results are promising and phase III trials are underway with a fast track granted by the Food and Drug Administration.
Steroids in the CrosshairsRecently, especially regarding LN, the role of steroids has been questioned, particularly at high doses, for control of severe manifestations of SLE, based on a medium-to-long term unfavorable balance. A daily steroid-free therapeutic regimen for the induction and maintenance of remission in LN, referred to as “Rituxilup”, has gained certain traction in the literature and certain meetings. This novel proposal consists of a combination of methylprednisolone at onset added to rituximab and mycophenolate, the latter adjusted to serum levels. With this treatment protocol, the authors communicated very favorable results in an almost 3-year of follow-up observational study, with complete response in 72% of treated patients and a partial or complete response in 92%.12 However, up to 33% presented a relapse during the observation period. The study has numerous limitations among which an overrepresentation of membranous glomerulonephritis, which constitute no less than 44% of the sample, stands out. It is well known that these patients have a better prognosis than those affected by proliferative forms. In that way, in a multicentric, observational study of the Systemic Autoimmune Diseases of the Spanish Society of Rheumatology (EAS-SER), we were able to demonstrate that, after 10 years of follow-up, only 6% of patients with membranous LN (of a total of 150 patients) had advanced stage chronic renal disease.13 It is not the first time that a steroid-free treatment protocol is successful, as seen in the trial by the UCL group led by David Isenberg14; but the data is too preliminary and the follow-up too short to anchor a radical change in strategy.
Vitamin D in Patients With Systemic Lupus ErythematosusVitamin D is associated to modulating effects on different levels of the immune response. We know that its deficit is associated to self-reactivity and its correction favors T lymphocyte differentiation into regulatory subtypes with a reduction, for example, in TH17.15,16 Because most cohorts report a vitamin D deficit in patients with SLE,17 it might be possible that beneficial effects would accompany its correction. With this purpose in mind, the Johns Hopkins cohort initiated an observational study that included 1006 patients, supplementing those defined as having a deficit, proving, at 128 weeks, changes parallel to disease activity (in terms of reduction, measured by PGA or SELENA-SLEDAI) and even in proteinuria, with an increase in vitamin D levels, being significantly higher (though modestly) in those patients that started at levels <40ng/ml and for changes in plasma levels >20ng/ml.18 Because vitamin D supplements might offer other benefits to SLE patients, such as the reduction of the incidence for colon and rectal cancer or favorable effects on bone metabolism, some experts’ recommend the supplementation of patients with vitamin D levels under 30–40ng/ml.19 In any case, because long term safety is not completely elucidated and the fact that in certain mouse models of SLE, such as NZW, vitamin D may worsen nephritis, it is clear that randomized clinical trials are needed in order to confirm these potential benefits, before recommending their generalized us in patients with SLE.
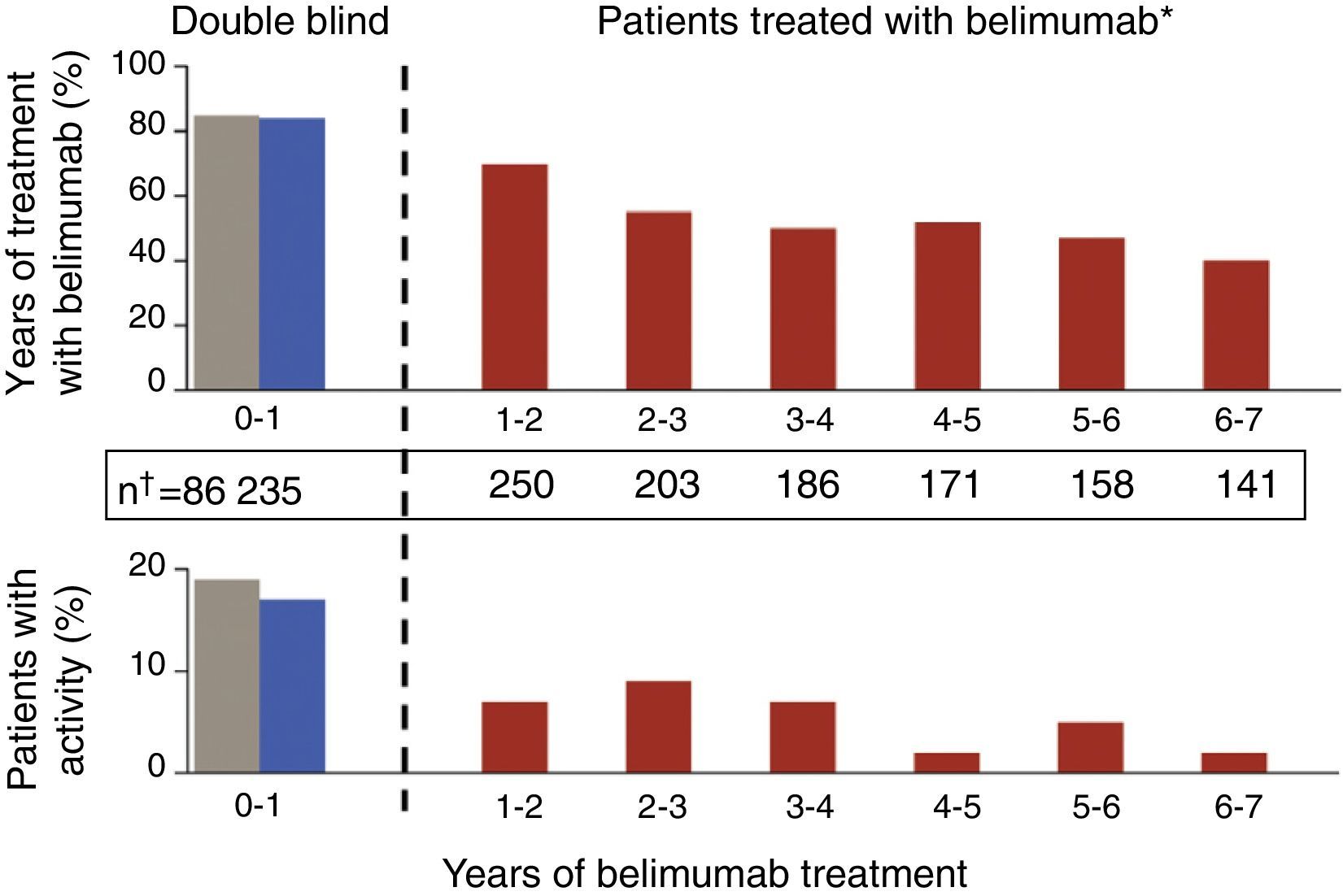
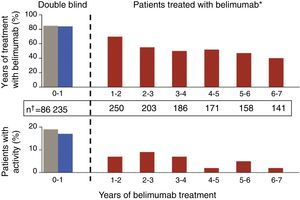
Update in Biological TherapyThe results of an open-label, phase II extension study with belimumab,20 with data from the follow-up of 1746 patients-year, have been published. In this study, which is the longest followed trial with a biological drug in SLE, patients were included if, at the investigators discretion, they were categorized as responders after a 24 week open phase. The percentage of SRI responders was seen to increase lightly and there was a gradual reduction in both mild and in moderate flares, as measured by the SELENA-SLEDAI flare index (SFI) (Fig. 2). In addition, there was a reduction in the mean dose of GC, around 50%, although few patients were capable of suspending them altogether. A limitation was that it was enriched with responders; in addition, given that the natural history of the disease assumes a tendency to a reduction in the frequency of flares with time, it is difficult to extract firm conclusions regarding the degree of efficacy. What is true is that approximately 50% of patients continued using belimumab after a mean of 7 years of follow up, a number that holds up well in comparison to survival with biologic therapy in other inflammatory rheumatic diseases such as rheumatoid arthritis. The most consistent data that studies of this kind can offer perhaps are those concerning safety and, in this respect, they can be qualified as good. The rate of annual adverse events remained stable, with that of infection being reduced as well as those related to infusion reactions, drastically after the first year. Only a small percentage developed hypogammaglobulinemia (stage III–IV of 1.1%–2.6%), without any serious infections.
Gradual reductions in flares of patients treated with 10mg/kg of belimumab, both severe as well as mild-moderate, throughout the extension phase of a phase II clinical trial in patients with SLE.
Another biological agent on the Launchpad for SLE is epratuzumab. The complete data of the EMBLEM, a phase IIb RCT that used epratuzumab, have been finally published (after an unusually long wait, given the favorable partial results seen at different meetings).21 This monoclonal antibody is directed against CD22, a molecule present on the membrane of the B cell that is closely related to the B cell receptor in such a way that its blockage leads to a down regulation of receptor mediated signaling. It is a treatment that leads to a certain degree of B cell ablation and modifies the expression of certain adhesion molecules, interfering with b cell circulation, among other effects it has on the immune system.22 EMBLEM is a multicenter RCT performed in patients with mild to moderate SLE, excluding LN or severe central nervous system affection, which had the objective of exploring the superiority of standard treatment and dose-ranging. Efficacy was measured in terms of the percentage of patients who reached a BICLA response, a new compound score, based fundamentally on BILAG and designed specifically for this study. At 24 weeks, the end of the study, favorable differences in the epratuzumab arm were seen, in several but not all of the doses. If these differences are confirmed, it is an intriguing effect, already seen with other biologics in SLE, which demands an explanation as to why at high doses, the beneficial effects seen at lower doses of the drug are lost. The phase III clinical development program, called EMBODY, includes 2 pivotal RCT and is in an advanced stage, awaiting the publication of results in the first trimester of 2015.
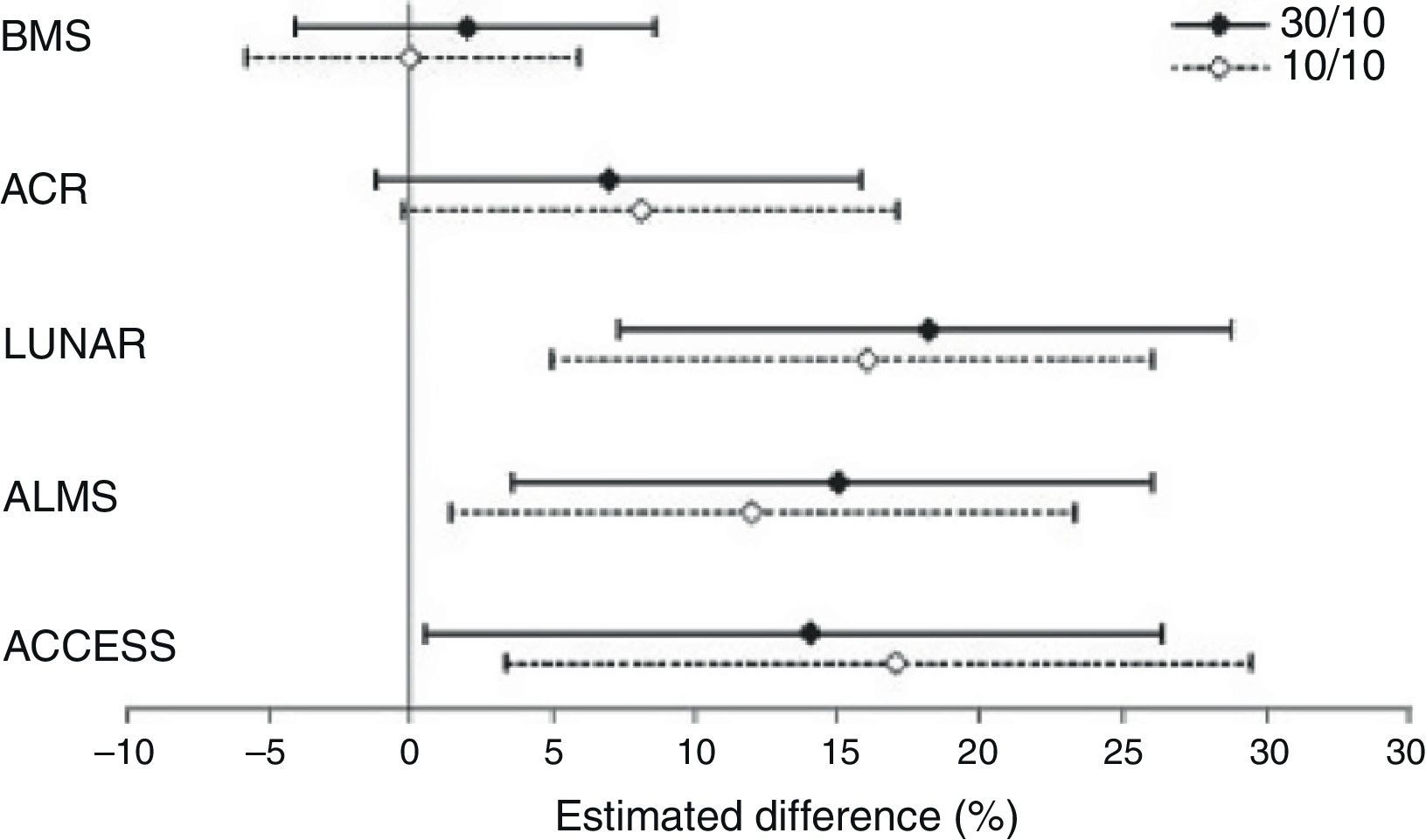
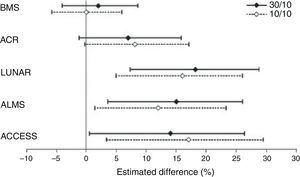
A study with abatacept in LN, initially considered as a failure, is noteworthy due to several reasons, among others because it illustrates the methodological difficulties related to RCT's in SLE and their implications in the analysis of results. This RCT, sponsored by Bristol-Myers Squibb, attempted to demonstrate the superiority of abatacept over placebo added to standard treatment, without achieving its primary objectives in none of the 2 doses studied.23 However, in a careful analysis of the conclusions, Wofsy et al. propose an intelligent exercise in post hoc result analysis, consisting of the use of different response scores designed for other RCT's (LUNAR, ALMS, etc.) only in those patients that complied with the inclusion criteria of the original trial for which the score was designed. As a result, with any of the response scores, significant favorable differences were seen for abatacept (Fig. 3). The only index that did not show differences was with the one designed for the study itself, the most demanding of them all.24 This study shows what seems to be a recurrent obstacle in many RCT's carried out to date in SLE: measurement instruments appear to have little sensitivity to change and may be reporting treatments as similar when they are not so.
Differences in the complete response rates in each abatacept treatment group, compared to standard treatment using different response definitions. ACCESS, abatacept and cyclophosphamide combination: efficacy and safety study; ACR, American College of Rheumatology recommendations; ALMS, Aspreva Lupus Management Study; LUNAR, Lupus Nephritis Assessment with Rituximab trial.
With the objective of facilitating the critical analysis of evidence and guiding clinical decisions in the complex field of biological therapy for SLE, the SER has recently published an expert concensus.25 The consensus was carried out using a group consensus methodology (RAND-UCLA) and included several systematic reviews (some of them, such as the one developed for clinimetry, never before published), definition of refractory disease and dependence on steroids. Additionally, as had to happen, it widely describes scientific off-label use of drugs, something common with this disease.
Antimalarial OptimizationNobody seems to doubt the efficacy of antimalarial for SLE and the universal need of their use for disease control, with multilevel potential benefits.26 With the purpose of contributing to optimize their efficacy, the group headed by Costedoat-Chalumeau proposed themselves the study of whether a strategy based on dose adjustments of hydroxicloroquine (HCQ) based on plasma levels was better, in terms of efficacy, than their traditional use at a fixed or weight adjusted dose.27 The hypothesis, without a doubt attractive, is fundamental in the well-known variability in the absorption of HCQ28 and in studies carried out in the past by the same group where they managed to prove a close relationship between disease activity and antimalarial levels.29 They employed a standardized tool for the capture of disease flares (SFI) but, unfortunately, did not find differences in incidence of flares between the two modalities of HCQ use. The short duration of the trial (7 months), in a population that was not selected according to risk of flaring, with a relatively low accumulation of the same, may be an explanation for this apparent failure. In any case, and given that the determination of HCQ plasma levels is not widely available, in this moment it is not advisable, to include in the “T2T” of SLE treatment.
I would like to finish by apologizing if I have not included studies that, in the opinion of others, might be relevant as well as for the bias toward clinical studies that are evidently applicable, something that inevitably stems from my condition as a clinical physician.
Ethical ResponsibilitiesProtection of people and animalsThe authors state that no experiments were carried out in humans or animals in this study.
Data confidentialityThe authors state that no patient data appears in this paper.
Right to privacy and informed consentThe authors state that no patient data appears in this paper.
Conflicts of InterestDr Rúa-Figueroa has acted as a consultant for Lilly and GSK.
Please cite this article as: Rúa-Figueroa Fernández de Larrinoa I. Lo mejor del año en lupus eritematoso sistémico. Reumatol Clin. 2015;11:27–32.