To determine whether an association exists between the presence of rheumatoid nodules and thickening of the intima-media and plaque of the carotid artery, which is evidence of atherosclerosis.
Materials and methodsObservational, cross-sectional study of 124 patients with rheumatoid arthritis from a University Hospital clinic from 2005 to 2006. We divided the patients into 2 groups, 62 with rheumatoid nodules and 62 without rheumatoid nodules, matched for age and sex. Medical history, erythrocyte sedimentation rate, anti-cyclic citrullinated peptide, rheumatoid factor, and a high resolution doppler ultrasound of the carotid arteries were performed.
ResultsWomen comprised 89.5% of the patients. The prevalence of a carotid plaque was 57% in our population. The presence of a plaque was associated with age, arterial hypertension and abdominal circumference. Average intima-media thickness (IMT) in patients with a plaque was 0.085cm (±0.02). There was no correlation between laboratory parameters and thickening of the intima-media of the carotid artery. Subcutaneous nodules were present in 33 (47%) of the 70 patients with a carotid plaque and in 29 (54%) of patients without a carotid plaque (p=.471).
ConclusionsWe did not find an association between rheumatoid nodules and the presence of a carotid plaque and thickening of the intima-media of the carotid in patients with rheumatoid arthritis.
Determinar si existe una asociación entre la presencia de nódulos reumatoides y el engrosamiento de la íntima-media y de placa de las arterias carótidas.
Materiales y métodosEstudio observacional, transversal de 124 pacientes con artritis reumatoide del Servicio de Reumatología de un Hospital Universitario desde 2005 a 2006. Se dividieron los pacientes en 2 grupos, 62 con nódulos reumatoide y 62 sin nódulos reumatoides, pareados por edad y sexo. Se realizó una historia clínica completa, velocidad de sedimentación globular, medición de anticuerpos antipéptidos cíclicos citrulinados, factor reumatoide y una ecografía doppler de alta resolución de las arterias carótidas.
ResultadosLas mujeres comprendieron el 89,5% de los pacientes. La prevalencia de al menos una placa en las carótidas fue del 57% en nuestra población. La presencia de placa carotídea estuvo asociada a la edad, hipertensión arterial y circunferencia abdominal. El grosor promedio de la íntima-media en pacientes con placa carotídea fue 0,085cm (±0,02). No hubo ninguna correlación entre los parámetros de laboratorio y el engrosamiento de íntima-media de la arteria carótida. Los nódulos subcutáneos estuvieron presentes en 33 (47%) de los 70 pacientes con placas de carótida y 29 (54%) de los pacientes sin una placa carótida (p=0,471).
ConclusionesNo hemos encontrado una asociación entre nódulos reumatoides y la presencia de placa y/o el engrosamiento de la íntima-media de carótidas en pacientes con artritis reumatoide.
Rheumatoid arthritis (RA) is a chronic, inflammatory, multisystemic, multifactorial, autoimmune disorder of unknown etiology that affects mostly diarthrodial joints.1 RA has a prevalence of 1–2% in the general adult population. The greatest incidence occurs between 35 and 45 years of age with predominance in women of 3–1. It presents as a chronic, bilateral, symmetrical erosive polyarthritis with diverse extrarticular manifestations that affect tissues and organs such as peripheral nerves, blood vessels, the lung, eyes, heart, and spleen with the presence of rheumatoid nodules, anemia, and symptoms of systemic disease. Three types of clinical course are recognized in this disease: type I, a self-limited process; type II, a polycyclic variant; and type III, the most frequent, which is progressive and deforming.2
Since the 1980s, RA has been known to lower life expectancy,3–8 with a mortality rate similar to that observed in patients with Hodgkin disease, diabetes mellitus, or cerebrovascular disease.4 Life expectancy can decrease 4–7 years in men and 3–10 years in women, but a decrease of up to 18 years has been reported.9 The mortality rate in patients with RA increases during the course of the disease with a tendency to accelerate after 15 years.10
The main causes of death are cardiovascular (37.4%), cerebrovascular (9.4%), and pulmonary diseases (10%), neoplasias (10.3%), and infections (15.2%).6,9,11–21 This information is from hospital22–28 and community studies20,21 that confirm that mortality is due mainly to cardiovascular disease. The incidence of cardiovascular disease in RA patients is independent of traditional cardiovascular risk factors.26,29–32 The underlying mechanism for this increase has not been elucidated. Cardiovascular mortality has been correlated with greater activity of the disease (increased erythrocyte sedimentation rate,33 severe extra-articular manifestations,34,35 rheumatoid factor seropositivity,36 C-reactive protein,36–38 and the presence of rheumatoid nodules). The inflammation that occurs in persons with RA has been suggested to accelerate the atherosclerotic process.30,36
Intima-media thickness of the wall of the common carotid artery measured by high resolution ultrasound is a safe, non-invasive, reproducible, clinically useful biomarker of early stage atherosclerosis that correlates with coronary involvement.39 This measure has greater predictive power than cardiovascular risk factors.40 Intima-media thickness in RA patients correlates with the duration and severity of the disease.33,36,41
The association between carotid atherosclerosis, confirmed by ultrasound, and inflammatory markers, such as erythrocyte sedimentation rate and C-reactive protein, is independent of age, sex, and cardiovascular risk factors such as hypercholesterolemia, systolic arterial hypertension,42 diabetes mellitus,43,44 and body mass index.24,25
In RA, rheumatoid nodules are considered a manifestation of more aggressive disease; patients with rheumatoid vasculitis are known to have twice the probability of developing rheumatoid nodules.45,46 Therefore, we considered it important to evaluate the correlation between atherosclerotic carotid disease and the presence of a specific extra-articular manifestation of RA, nodules. Rheumatoid nodules are tissue tumors that are usually located subcutaneously and that can be found in diverse organs, including the eye, lung, heart and brain. They are present in 20–40% of patients with rheumatoid arthritis and are more frequent on extensor surfaces of the limbs such as the olecranon. Nodules vary in consistency from a soft, amorphous, completely mobile mass to a hard mass firmly attached to the periosteum. They differ in size from a few millimeters to several centimeters and are usually painless. Their histologic appearance is considered characteristic, although not completely pathognomonic. Development of nodules is mediated through effects on small arterioles and the resultant activation of complement and terminal vasculitis.
In addition, another interesting point is the effect of anti-tumor necrosis factor alpha therapy and the methotrexate on the progression of atherosclerosis in RA patients through the determination of carotid IMT by ultrasound. In fact, several studies have shown a reduction of carotid IMT in patients with active RA receiving this medication.47,48 Therefore, this image method could be a very useful tool for regular assessment, monitoring and management of cardiovascular risk in this population.
Patients and methodsThe participants were patients who met at least 4 American College of Rheumatology49 criteria for RA and who also attended the Rheumatology Service of the Hospital Universitario “Dr. Jose Eleuterio Gonzalez” of the Universidad Autónoma de Nuevo León from August 2005 to July 2006. The selection of patients with rheumatoid nodules was not based on a consecutive sequence of attendance to the clinic, but on an intentional search for the presence of nodules. Patients with nodules were matched with RA patients that did not have rheumatoid nodules according to age and sex.
Enrolled patients were greater than 16 years of age and provided informed consent for participation. Pregnant patients or those with a history of carotid surgery were excluded.
Clinical evaluation included a complete medical history with determination of active rheumatoid arthritis inflammation using the criteria previously mentioned50; we also documented the presence of extra-articular manifestations. In order to find out a possible association between IMT and antirheumatic therapy, including methotrexate, biologic therapy (anti-TNF blockers), antimalarials, and steroids, we classified the time of exposition to antirheumatic drugs in categorical variable (0=none, 1=1–6 months, 2=7–12 months, 3=1–3 years, 4=3–5 years, 5=5–7 years, 6=7–10 years, 7=>10 years).
Patients filled out a validated self-applied questionnaire in Spanish, the Modified Health Assessment Questionnaire (MHAQ).27 The following laboratory tests were performed: complete blood count, liver function tests, urinanalysis, blood chemistry, and lipid profile. Also, C-reactive protein, erythrocyte sedimentation rate, rheumatoid factor, and anti-cyclic citrullinated peptide (anti-CCP) antibody titer were measured.
To measure C-reactive protein, we used the Lafon Protex-CR (Laboratorios Lafon, S.A. de C.V., Mexico) latex agglutination technique, which determines and quantifies C-reactive protein in serum by an indirect method.51 A test was positive when macroscopic agglutination, and a clear plate bottom comparable to a positive control at a dilution of 1:40, equivalent to a sensitivity of 1.0mg/dL, occurred.
Erytrosedimentation rate (ESR) was determined using the Winthrobe method. The upper limit of normal adjusted for age was considered a significant result; for men it was obtained by dividing the patient's age by two, and in women, age plus ten divided by two.51
The Lafon Reumatex kit, in which the globulin latex reagent (polystyrene particles sensitized with human gamma-globulin) agglutinates in the presence of serum with the rheumatoid factor adequately diluted, was used to measure rheumatoid factor. A test was positive when macroscopic agglutination occurred with the formation of clumps at a dilution of 1:20.
The Axis-Shield Diastat (Axis Shield Diagnostics Ltd., UK) was used to determine anti-cyclic citrullinated peptide antibodies. This is a semiquantitative/qualitative enzyme-linked immunosorbent serologic assay (ELISA) for detecting specific IgG antibodies in human serum or plasma.51 The reference range considered positive was >5U/ml.
The measurement of IMT was performed with the patient in supine position with a high-resolution ultrasonography (General Electric Logic 9) of both common carotid artery below its bifurcation and of the proximal internal carotid artery with a 14MHz linear transducer. The arterial wall segments were assessed in a longitudinal view.52 The measurement of the IMT was performed bilaterally on the far wall of the common carotid artery taking into account for the analysis the thickest. Plaque thickness was expressed in centimeters. Measurements of intima-media thickness were obtained according to a standardized protocol.25 One radiologist performed all studies without clinical information about the patients.
Carotid intima-media thickness was evaluated according to the Mannheim Carotid Intima-Media Thickness Consensus (2004–2006),52 in which a plaque is defined as the focal structure that invades the lumen of the artery by at least 0.5mm or 50% of the value of intima-media thickness, or when thickness is equal to or greater than 1.5mm when measured from the adventitia-media interphase to the intima-arterial lumen interphase.52
Statistical analysisThe number of patients needed for study was 124, with a β value of 0.2 and an α value of 0.05, so the group was divided into 62 with rheumatoid nodules (n) and 62 without nodules.
We used a comparative univariate analysis with Student's t-test for normally distributed variables, χ2 and Fisher's exact test for non-normally distributed and binary variables, and Mann–Whitney and Kruskal–Wallis U-tests for non-parametric variables. We also did multivariate analysis with linear regression coefficient with variables that had p<0.2 and those were age, sex, hypertension, systolic blood pressure, waistline, thickness of the intima-media (TIM), hypercholesterolemia. Regarding the association between time of exposition of antirheumatic therapy and IMT we used a Spearman's rank correlation coefficient.
Statistical significance was defined at p<0.05 for univariate and multivariate analysis.
The study protocol was evaluated and approved by the Research and Ethics Committee of the Facultad de Medicina and Hospital Universitario “Dr. Jose Eleuterio Gonzalez” of the Universidad Autonoma de Nuevo Leon (Registration no. RE-05-011). The study was financed by the Programa de Apoyo a Investigacion Científica y Tecnológica (PAICyT 2006) of the Universidad Autonoma de Nuevo Leon, code number SA1191-05.
ResultsOf the 124 patients examined in this study, 111 were women (89.5%) and 13 were men (10.5%), with an average age of 55.5 years (±13.1).
The average duration of RA in the study population was 12.4 years (±9.7). Rheumatoid factor was positive in 92 patients (74%); 90 were anti-CCP positive (72%).
Fifty-five patients (44%) had hand deformities, 44 (35%) active disease and 80 (65%) were in remission. There was no difference between the groups regarding the use of different antirheumatic drugs including time of exposition (Table 1).
Characteristics of patients with rheumatoid arthritis.
| Characteristic | Whole RA group | RA patients w/o nodules | RA patients with nodules | p |
| Number of patients | 124 | 62 | 62 | NS |
| Men (n) | 13 | 7 | 6 | NS |
| Women (n) | 111 | 55 | 56 | NS |
| Age (years), mean (SD) | 55.5 (10.8) | 56.97 (12.65) | 54.03 (13.45) | NS |
| Time of evolution of RA, mean (SD) | 12.38 (9.71) | 10.94 (9.37) | 13.84 (9.92) | NS |
| Hand deformities, n (%) | 54 (44) | 17 (27.4) | 37 (59.7) | 0.001a |
| Active disease, n (%) | 44 (35) | 15 (24.2) | 29 (46.8) | 0.014a |
| Disease in remission, n (%) | 80 (65) | 47 (75.8) | 33 (53.2) | 0.009a |
| Positive rheumatoid factor, n (%) | 92 (74) | 39 (62.9) | 53 (85.5) | 0.02a |
| Positive anti-CCP, n (%) | 90 (72) | 35 (56.5) | 55 (88.7) | 0.008a |
| Methotrexate | 88 (71%) | 46 (74%) | 42 (68%) | 0.429 |
| TEc | – | – | – | 0.507 |
| Antimalarial drugs | 51 (41%) | 28 (45%) | 23 (37%) | 0.362 |
| TEc | – | – | – | 0.283 |
| Biological therapy | 27 (21.7%) | 14 (23%) | 13 (21%) | 0.828 |
| TEc | – | – | – | 0.688 |
| Steroids | 68 (55%) | 39 (63%) | 29 (47%) | 0.071 |
| TEc | – | – | – | 0.075 |
| Hypertension, n (%) | 36 (29) | 21 (33.9) | 15 (24.2) | NS |
| Dyslipidemia, n (%) | 19 (15) | 13 (21) | 6 (9.7) | NS |
| BMI (kg/m2), mean (DS) | 27.8 (4.8) | 28.49 (5.26) | 27.1 (4.2) | NS |
| Waistline circumference (cm), mean (DS) | 88.4 (14.3) | 90.58 (15.5) | 86.21 (12.73) | NS |
| Tobacco use, n (%) | 49 (39.5) | 28 (45.16) | 21 (33.9) | NS |
| IMTb(cm), mean (DS) | 0.07654 (0.020) | 0.076 (0.019) | 0.077 (0.022) | NS |
| Patients with a plaque, n (%) | 70 (57) | 37 (59.7) | 33 (53.2) | NS |
For the whole group, carotid intima-media thickening had a range of 0.04–0.17cm, with an average of 0.08cm (±0.02). The presence of an atherosclerotic plaque in the population was 57%.
There were 70/124 (56.45%) patients with a carotid plaque with an average age of 62 years, while the average age of the 54 patients without a carotid plaque was 47 years (p=0.001). Of the patients with a plaque, 62 (89%) were women, while 49 (91%) were women without a plaque (p=NS).
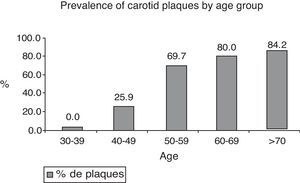
In the age group of 30–39 years, no carotid plaque was found; in the group 40–49 years of age, it was detected in 25%; 50–59 years, 69.7%; 60–69 years, 80%; and in patients greater than 70 years, 84.2% (Fig. 1).
The average duration of disease in patients with a carotid plaque was 13.4 years (±10.7) while in those without a carotid plaque was 11 years (±8.2). In 33 (47%) of the 70 patients with a carotid plaque, the presence of subcutaneous nodules was found and in patients without a carotid plaque the nodules were found in 29 (54%) (p=0.471). There was no association between thickening of the intima-media or plaque and the clinical variables of rheumatoid arthritis, such as disease duration, joint deformity, morning stiffness, number of painful joints, number of inflamed joints, or rheumatoid nodules.
There was also no association between erythrocyte sedimentation rate, C-reactive protein, rheumatoid factor, anti-CCP antibodies, and the patients with or without a carotid plaque.
Among the traditional cardiovascular risk factors, only hypertension, as well as waistline circumference, showed an association with the presence of a carotid plaque.
Average intima-media thickness in patients with a plaque was 0.086cm (±0.02).
One-third of the studied population had an average age of 64.8 years (±9.3) (p<0.001) and this correlated with the presence of thickening of the carotid intima-media (p<0.001).
We were not able to find an association between the time of exposition to the antirheumatic drugs and IMT or carotid plaque; however, the correlation analysis showed a trend for the time of exposition to methotrexate (p=0.064) and the use of anti-TNF (p=0.079) and a lower IMT at the common carotid artery.
DiscussionContrary to our hypothesis, in our population of 124 patients with RA, there was no association between the presence of rheumatoid nodules and ultrasonographic evidence of carotid atherosclerosis (intima-media thickness and plaque).
On the other hand, it was notable in this study that the prevalence of carotid plaque in our population was 57%, which is greater than the 44% found by Roman53 in New York, in an Anglo-Saxon patient population with rheumatoid arthritis, and higher than the 15% found in control subjects without rheumatoid arthritis in this same population.
As was expected, age, a known cardiovascular risk factor, was the variable that was most associated with the presence of a carotid plaque and to thickening of the intima-media. The data of this population are comparable with world statistics, which indicate that an increase in the prevalence of carotid plaque is directly proportional to the age of the individual.
Besides age, two other factors that correlated with the presence of a plaque were hypertension and waistline circumference, which are also factors described for cardiovascular disease (Table 2).
Cardiovascular risk factors found in the univariate analysis of independent variables vs. the presence of carotid plaque.
| Variable | Carotid plaque present, n=70 | Carotid plaque absent, n=54 | p |
| Diabetes mellitus, n (%) | 9 (13) | 7 (13) | NS |
| Hypertension, n (%) | 28 (40) | 8 (15) | 0.002c |
| Systolic blood pressure (mmHg) | 128.5 (SD 16.7) | 120.2 (SD 12.5) | 0.011 |
| Diastolic blood pressure (mmHg) | 80.4 (SD 9.4) | 77.6 (SD 7.8) | NS |
| Hypercholesterolemia, n (%) | 29 (41) | 19 (35) | NS |
| Tobacco use, n (%) | 28 (40) | 21 (39) | NS |
| BMIa (kg/m2) | 27.9 (SD 4.8) | 27.7 (SD 4.8) | NS |
| Waistline circumference (cm) | 90.7 (SD 13) | 85.2 (SD 15.5) | 0.017c |
| IMTb (cm) | 0.08564 (SD 0.02041) | 0.06453 (SD 0.01278) | <0.001c |
With regard to clinical manifestations of rheumatoid arthritis, we did not find any parameter associated with a greater prevalence of plaque or intima-media thickness, although it is worthy to point out that duration of the disease tended to be greater in the upper third with regard to intima-media thickness.
A possible explanation for the lack of association between rheumatoid nodules and carotid atherosclerosis is that the pathogenic mechanisms involved in their formation, contrary to what was previously believed, do not have a significant vascular component, and are most probably due to other factors, including the idea that it is started by local trauma, which permits the release of circulating immune complexes and rheumatoid factors from small vessels of connective tissue, and that the macrophages and local monocytes activate and release cytokines and angiogenic agents that determine a chronic inflammatory process.
It could be considered that with a greater number of patients in each subgroup the difference could have been more notable. This option, however, has been discarded, due to the statistical power of the studied sample.
With regard to the greater prevalence of carotid plaque in our population in comparison with Roman, this could be due to genetic or ethnic factors, since in their report the majority of patients were Anglo-Saxon, and very few were of Hispanic origin. It is well known that some other chronic degenerative diseases, such as type 2 diabetes mellitus, have a greater incidence in Mexican population with regard to non-Hispanic whites.54
Other potential factors that can contribute to this difference could be related to educational and sociocultural aspects, which would have as a consequence poor compliance to treatment, and therefore, poor control of the inflammatory activity of RA in our population, as well as a lower use in our setting of biological agents (undemonstrated data); drugs that not only show an important ability to induce remission in rheumatoid arthritis, but that also decrease cardiovascular complications in patients with RA.55–57
Recently, the presence of anti-CCP antibodies and RF IgM has been associated with endothelial dysfunction in patients with rheumatoid arthritis independent of other cardiovascular risk factors in RA patients, but it was not evaluated in our work.58
Currently, there is a consensus about the extreme usefulness of the carotid ultrasound in detecting subclinical atherosclerotic findings in patients with long-term RA without clinically evident atherosclerotic disease, as showed by Gonzalez-Juanatey et al.59 They were able to demonstrate greater carotid IMT in a population of 47 patients comparing with controls, and the relationship between carotid plaques and a longer disease duration and more extraarticular manifestations. Age at the time of study and disease duration were the best predictive factors for the development of atherosclerotic disease.59
Of paramount importance is the potential utility of carotid ultrasonography in the prognosis value of subclinical atherosclerosis in RA and its application in monitoring the effect of new biological drugs in the evolution of atherosclerotic disease.48 Carotid IMT has been used as a marker of progression of atherosclerosis in RA patients undergoing treatment with anti-tumor necrosis factor alpha blockers due to severe disease, and refractory to methotrexate.47 In a study response to anti-tumor necrosis factor alpha blockade was associated with reduction of carotid intima-media thickness in patients with active rheumatoid arthritis.48
In our study, however, we did not find an effect of the use of methotrexate, anti-TNF blockers, antimalarials or steroids, on the IMT, even though there was a trend to lower IMT with the first two of them.
Interestingly, an association between the magnitude and chronicity of the inflammatory response, determined as the mean C-reactive protein in long-standing RA patients without clinically evident cardiovascular disease and the carotid IMT values has also been reported.60
ConclusionsWe did not find an association between the presence of rheumatoid nodules and thickening of the intima-media, and plaque of the carotid artery as evidence of atherosclerosis in patients with rheumatoid arthritis.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe study protocol was evaluated and approved by the Research and Ethics Committee of the Facultad de Medicina and Hospital Universitario “Dr. Jose Eleuterio Gonzalez” of the Universidad Autonoma de Nuevo Leon (Registration no. RE-05-011). Enrolled patients were older than 16 years of age and provided informed consent for participation. Pregnant patients or those with a history of carotid surgery were excluded.
FundingThe study was financed by the Programa de Apoyo a Investigacion Científica y Tecnológica (PAICyT 2006) of the Universidad Autonoma de Nuevo Leon, code number SA1191-05. Clinical evaluation included a complete medical history, self-applied questionnaire in Spanish, routine lab tests, and Bilateral high-resolution ultrasonography was performed of the common carotid artery.
Conflict of interestNo conflict of interest.
We thank Dr. Gerardo Ornelas Cortinas for their collaboration in this work as radiologist; and to Dr. Sergio Lozano Rodríguez for the paper traduction.