Chronic fatigue syndrome (CFS) is a chronic condition that predominantly affects women. To date, there are few epidemiologic studies on CFS in men. The objective of the study was to assess whether there are gender-related differences in CFS, and to define a clinical phenotype in men.
Patients and methodsA prospective, cross-sectional cohort study was conducted including CFS patients at the time of diagnosis. Sociodemographic data, clinical variables, comorbid phenomena, fatigue, pain, anxiety/depression, and health quality of life, were assessed in the CFS population. A comparative study was also conducted between genders.
ResultsThe study included 1309 CFS patients, of which 119 (9.1%) were men. The mean age and symptoms onset were lower in men than women. The subjects included 30% single men vs 15% single women, and 32% of men had specialist work vs 20% of women. The most common triggering factor was an infection. Widespread pain, muscle spasms, dizziness, sexual dysfunction, Raynaud's phenomenon, morning stiffness, migratory arthralgias, drug and metals allergy, and facial edema were less frequent in men. Fibromyalgia was present in 29% of men vs 58% in women. The scores on physical function, physical role, and overall physical health of the SF-36 were higher in men. The sensory and affective dimensions of pain were lower in men.
ConclusionsThe clinical phenotype of the men with CFS was young, single, skilled worker, and infection as the main triggering agent. Men had less pain and less muscle and immune symptoms, fewer comorbid phenomena, and a better quality of life.
El síndrome de fatiga crónica (SFC) es una entidad que afecta predominantemente a las mujeres, con escasos estudios epidemiológicos en los hombres. El objetivo fue evaluar si existen diferencias de género en el SFC y definir el perfil clínico en el hombre.
Pacientes y métodoEstudio de cohorte transversal prospectivo de inclusión de pacientes con SFC en el momento del diagnóstico. Se evaluaron datos sociodemográficos, clínicos, fenómenos comórbidos y evaluación de la fatiga, dolor, ansiedad/depresión y calidad de vida a través de cuestionarios. Se realizó un estudio comparativo de las variables entre género.
ResultadosSe estudió a un total de 1.309 pacientes con SFC, de los cuales 119 (9,1%) fueron hombres. La edad media y de inicio de los síntomas de los hombres fueron menores que en las mujeres. El 30% eran hombres solteros, vs el 15% de mujeres, y el 32% tenían un trabajo especializado vs el 20% en mujeres. El desencadenante más frecuente fue el infeccioso. El dolor generalizado, las contracturas musculares, los mareos, la disfunción sexual, el fenómeno de Raynaud, la rigidez matutina, las artralgias migratorias, las alergias a fármacos y metales, así como el edema facial, fueron menos frecuentes en los hombres. Se presentó fibromialgia en 29% de los hombres vs 58% de las mujeres. Los fenómenos comórbidos fueron menos frecuentes en los hombres. Las puntuaciones en la función física, el rol físico y la salud física global del SF-36 fueron más altas en los hombres. Las dimensiones sensorial y afectiva del dolor fueron inferiores en los hombres.
ConclusionesEl perfil clínico del hombre fue el de un paciente más joven, soltero, con trabajo especializado y con un desencadenante infeccioso. Los hombres presentaron menor dolor, menor sintomatología muscular e inmune, menor número de fenómenos comórbidos y mejor calidad de vida.
Chronic fatigue syndrome (CFS) is a multisystem condition of unknown cause that affects mostly young adults, with ages ranging between 20 and 40 years; the male to female ratio is 1:4 in some series.1,2 The prevalence is estimated to be between 0.2% and 2.6% of the general population.3,4 Chronic fatigue syndrome should be suspected in patients with signs and symptoms of inexplicable fatigue that have persisted for at least 6 months and do not improve with rest. The fatigue should be accompanied by 4 or more of the following symptoms: impaired short-term memory and concentration, odynophagia, tender cervical or axillary lymph nodes, muscle pain, arthralgia with no signs of inflammation, headache of a new kind, with different characteristics or severity, unrefreshing sleep, and exhaustion lasting more than 24h after exercise. All these symptoms should be indicative of a serious functional disorder, as proposed by the international diagnostic criteria established by the Centers for Disease Control (CDC) in Atlanta, Georgia, in 1994.5 In 2003, a new case definition for CFS was proposed with the intention of excluding psychiatric cases, as was put forward in the Canadian consensus document on CFS.6 The Canadian criteria are useful and complementary to the CDC criteria, and enable us to study the symptoms in clusters (neurological, muscle, cognitive, neurovegetative and immunological). In 2011, these criteria were updated, and postexertional exhaustion was proposed as a hallmark of the disease.7 Chronic fatigue syndrome is associated with different comorbid phenomena (sicca syndrome, regional myofascial pain syndrome, anxiety-depressive disorders, plantar fasciitis, degenerative or mechanical disk disease, tendinopathy of the shoulder and fibromyalgia (FM),8,9 that are more prevalent in CFS patients than in non-CFS individuals.10 Because of the smaller number of men with CFS—in our experience, the ratio of men to women is 1:99—epidemiological and clinical studies in CFS have basically been carried out in women, and there are few studies on the profile of men with CFS. The differences between men and women in terms of health are partly determined by biological differences that, in addition to reproductive functions, involve genetic, hormonal and neurometabolic factors. At the present time, the most widely accepted hypothesis for the pathogenesis of CFS characterizes it as a genetic-based process, with different triggering factors and subsequent neuroimmunological and immunoinflammatory dysfunction, which would produce the different symptoms observed in patients.11 There are clinical phenomena in CFS that are also observed in immunoinflammatory diseases that are more prevalent in women (systemic lupus erythematosus, multiple sclerosis, rheumatoid arthritis, Sjögren's syndrome and irritable bowel syndrome),12 with improvement in the symptoms during pregnancy and worsening during menstruation and childbirth. For all the above, it is logical to hypothesize that CFS will affect more women than men, although, at the present time, the clinical phenotype that differentiates men from women has not been defined. The objective of this study was to evaluate the clinical characteristics of men with CFS and compare them with those of women in order to determine whether there are gender differences in patients with CFS and, thus, to establish a differential clinical profile, with implications involving the prognosis and therapeutic management.
Patients and MethodsWe studied 1309 patients who were diagnosed with CFS in the chronic fatigue unit of Hospital Universitario Vall d’Hebron in Barcelona, Spain, on the basis of the diagnostic criteria5 and who, after receiving the diagnosis, agreed to be included in a database. Table 1 shows the inclusion and exclusion criteria for the study. The design was that of a prospective, cross-sectional, cohort study in which patients were enrolled at the time of diagnosis. We evaluated the influence of gender on the clinical presentation of the disease, the presence of associated comorbidities, symptom scores and questionnaires administered. The majority of the patients had been referred to the unit from the first level of care or specialized care, from all of the Spanish autonomous communities, because they showed compatible clinical signs. They were consecutively enrolled from January 2008 to May 2011. The interviews were conducted by 2 internists skilled in the diagnosis of the disease. All of the patients were requested to give their written informed consent and the study was approved by the ethics committee of the Hospital Universitario Vall d’Hebron. Sociodemographic data (age, sex, marital status, profession, employment status and level of education) were gathered for all the participants in the study, and are shown in Table 2. These patients were asked about the features of their fatigue (form and moment of onset, course and duration) and those of their pain (age at onset, length of time with continuous pain). The clinical interview was structured according to clusters of symptoms (muscle, cognitive, neurological, autonomic/neurovegetative and immunological) defined by the Canadian criteria for CFS.7 It took into account the presence of recurrent headache and sleep disorders (unrefreshing sleep, insomnia, nightmares and poor quality of sleep) and associated comorbidities (restless leg syndrome, sleep paralysis and mild sleep apnea-hypoapnea syndrome). The patients were asked about the existence of FM, defined according to the American College of Rheumatology13; sicca syndrome, defined as the presence of mouth dryness plus eye dryness, and demonstrated by the Schirmer test; regional myofascial pain syndrome, defined as pain, stiffness and claudication of the temporomandibular joint, according to the criteria of Travell and Simons; anxiety disorders (generalized anxiety and distress disorders), according to the definition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV); plantar fasciitis; degenerative or mechanical diseases of the vertebral column; and tendinopathy of the shoulder. Data was also gathered on the presence of osteoporosis, dyslipidemia, endometriosis and multiple chemical sensitivity. The symptoms fatigue, pain, anxiety/depression and quality of life were quantified in all the patients by means of self-administered questionnaires. The fatigue impact scale (FIS)14 was chosen to assess fatigue. This version, FIS 40, includes 3 subscales that examine the perceived impact of fatigue on cognitive, physical and psychosocial functioning, measured on the basis of 10, 10 and 20 items, respectively, where each item was rated from 1 to 4. To evaluate pain, the McGill questionnaire15 evaluates quantitative (intensity) and qualitative aspects of pain (sensory and emotional). Each of the descriptive terms is assigned a number or range, which makes it possible to obtain a score according to the terms selected, from which the so-called “pain rating index” (PRI) is obtained. This score reflects the way in which the patients rate their own experiences with pain, enabling the researcher to assess the influence of emotional and sensory factors on these experiences. It also has a section in which the patients reflect the intensity of the pain they feel. In the quantification of anxiety/depression, the hospital anxiety and depression scale (HADS)16 was employed. It consists of 14 statements referring to symptoms of anxiety and depression, in which the frequency or intensity is rated on a 4-point scale (with values ranging from 0 to 3). The total score for each subscale is obtained by adding up the items, with a range from 0 to 21. Finally, the SF-36 questionnaire17 was used to evaluate quality of life. It is a generic scoring system that provides the profile of the health status, based on 36 questions that explore 8 dimensions of health status (physical functioning, physical role, body pain, general health, vitality, social functioning, emotional role and mental health).
Criteria for Inclusion and Exclusion of Patients With Chronic Fatigue Syndrome.
| Inclusion criteria | 1. Patients over 18 years of age of both sexes 2. Patients with CFS according to established diagnostic criteria5 from the chronic fatigue unit, Hospital Universitari Vall d’Hebrón, Barcelona, Spain 3. Patients who willingly give their written informed consent to participate in the study |
| Exclusion criteria | 1. Patients who are participating in another study of the same or a different nature, or have been within the 30 days prior to inclusion 2. Any individual who the investigator considers to be unable to follow the instructions of the study or complete the self-administered questionnaires 3. Individuals who do not give their written informed consent to participate in the study |
Sociodemographic Data of the Study Patients.
| Men (n=119) | Women (n=1190) | Total (n=1309) | P | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age, years (mean±SD) | 43±11.4 | 47.9±10.1 | 47.4±10.3 | <.001** | |||
| Marital status | .002* | ||||||
| Married | 70 | 58.8 | 821 | 68.9 | 891 | 68.1 | |
| Single | 37 | 31.1 | 194 | 16.3 | 231 | 17.6 | |
| Separated | 11 | 9.2 | 148 | 12.4 | 159 | 12.1 | |
| Widowed | 1 | 0.8 | 27 | 2.2 | 28 | 2.1 | |
| Profession | <.001** | ||||||
| Unskilled worker | 52 | 43.6 | 511 | 42.9 | 563 | 42.9 | |
| Skilled worker | 38 | 31.9 | 237 | 19.9 | 275 | 21.0 | |
| Office worker | 10 | 8.4 | 239 | 19.8 | 249 | 18.8 | |
| Teacher | 1 | 0.8 | 43 | 3.6 | 44 | 3.4 | |
| Self-employed | 2 | 1.7 | 16 | 1.3 | 18 | 1.4 | |
| Liberal arts | 10 | 8.4 | 63 | 5.2 | 73 | 5.5 | |
| Craftsperson-artist | 1 | 0.8 | 10 | 0.8 | 11 | 0.8 | |
| Student | 5 | 4.2 | 15 | 1.2 | 20 | 1.5 | |
| Homemaker | 0 | 0 | 36 | 4.7 | 56 | 4.3 | |
P: chi-squared test (comparisons by sex), Kruskal–Wallis nonparametric test (comparison of means).
A descriptive analysis of the sample was carried out by applying absolute and relative frequencies in the case of categorical variables, and using measures of central tendency and dispersion for the continuous variables. The internal consistency of the items was calculated on the basis of Cronbach's alpha to estimate the reliability related to the muscle, cognitive, neurological and immunological symptoms and the neurovegetative dysfunction, for the purpose of evaluating the possibility of obtaining consistent results in successive measurements of a given phenomenon. If the value for alpha was greater than 0.7, the data were considered to be acceptable and it would be possible to work in a single dimension, which would be the sum of the items of each group of symptoms. The values of the categorical variables between men and women with CFS were compared with the chi-squared test of independence, and the t-test for independent samples was used for the comparisons between continuous variables. The type I error (α) was set at 5%. The statistical analysis was performed using R software (The R Foundation for Statistical Computing).
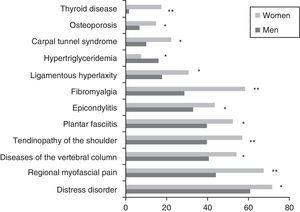
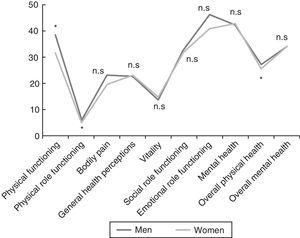
ResultsA total of 1309 patients (91% women vs 9% men) with an overall mean age of 47.4±10.3 years were enrolled. The men were diagnosed at an earlier age than the women (43.0±11.4 vs 47.9±10.1 years; P<.001). Concerning marital status, the percentage of unmarried men was higher than that of women (31.1 vs 16.3%; P=.002). More men than women were categorized as skilled workers (31.9 vs 19.7%; P<.001). There were no statistically significant differences with respect to the level of education or employment status. The comparison of the demographic variables of the 2 sexes are shown in Table 3. A total of 461 patients (35.2%) had family histories of CFS, FM or immunologic, rheumatologic or thyroid diseases; the prevalence of CFS was 11.8%, with no significant differences between sexes. In all, 76.4% of the patients had some personal history of fatigue, chronic pain, psychopathological symptoms prior to the onset of pain or fatigue, or immunologic diseases. The men reported less chronic pain than the women (18.5% vs 27.9%; P=.027), but there were no statistically significant differences in any of the other conditions reported in their disease histories. The assessment of the presence of a triggering factor prior to the onset of symptoms revealed gender differences: men reported an infectious process more frequently than women (26.9% vs 13%; P<.001) and fewer men than women recalled a stressful life event (17.6% vs 21.8%; P<.001). The fatigue developed gradually in 66% and was continuous in 83.9% of the patients, 88.1% of whom reported that it had begun to worsen from the very onset. The men were younger at the onset of fatigue (34.5±12 years; P=.016) and had a shorter history of pain (90.2±70.1 months; P=.036) than the women. The numbers of men and women who had recurrent headache were similar (86.4% vs 85.4%). In all, 98.7% of the patients reported unrefreshing sleep, with similar percentages for both sexes. Nightmares and insomnia were less common in the men than in the women (33.6% vs 58.8%; P=.012). There were no statistically significant gender differences in the assessment of associated phenomena (restless leg syndrome, sleep paralysis and sleep apneas). Of all the clusters of symptoms studied (muscle, cognitive, neurological, neurovegetative and immunological), only those composed of cognitive, neurological and neurovegetative symptoms had an internal consistency with a Cronbach's alpha higher than 0.70. Thus, those results could be worked with in a single dimension, stratifying the disease as mild, moderate or severe, depending on the number of symptoms. There were no statistically significant differences between sexes in either cognitive or neurological symptoms. Hypersensitivity to smells was more common among women (68.2%; P=.002). In the group of neurovegetative symptoms, dizziness (69.7%; P<.001) and sexual dysfunction (69.7%; P=.023) were less prevalent in men. The greatest differences between the sexes were found in the immunological and muscle symptoms. In the immunological symptoms studied, there were gender differences with respect to Raynaud's phenomenon (19.3% vs 27.9%; P=.045), generalized morning stiffness (76.5% vs 83,7%; P=.045), migratory arthralgia (79% vs 86.4%; P=.028), allergy to drugs (16% vs 24.8%; P=.032), allergy to metals (6.7% vs 17.1%; P=.003) and facial edema (2.5% vs 7.7%; P=.037). On the other hand, of the muscle symptoms, generalized pain (78.2% vs 90.9%; P<.001), difficulty in making delicate movements due to pain (77.3% vs 86.1%; P=.010) and muscle cramps (83.2% vs 89.6%; P=.034) occurred less frequently in the men. Fig. 1 shows the rates of comorbidities in the study patients according to sex. Fibromyalgia, musculoskeletal conditions (regional myofascial pain) and thyroid disorders were more common in the women, whereas hypertriglyceridemia was detected more frequently in the men. No statistically significant gender differences were observed in the FIS 40 or HADS scores. In the SF-36 questionnaire, the men had better scores on the subscales concerned with physical functioning (38.4±23.5; P=.002), physical role functioning (6.2±15.7; P=.034) and general physical health (27±6.8; P=.035) (Fig. 2). Statistically significant differences were observed in the McGill pain questionnaire: in the sensory and emotional dimensions, the men's scores were lower than the women's (16±8.3 vs 18.6±8.0; P=.005 and 6.4±3.8 vs 7.2±3.9; P=0.024, respectively). No significant gender differences were observed in the intensity of the pain.
Family History, Personal History and Apparent Triggers in the Study Patients.
| Men (n=119) | Women (n=1190) | Total (n=1309) | P | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Family history | NS | ||||||
| Chronic fatigue syndrome | 20 | 16.8 | 135 | 11.3 | 155 | 11.8 | |
| Fibromyalgia | 18 | 15.1 | 127 | 10.7 | 145 | 11.1 | |
| Immune diseases | 5 | 4.2 | 97 | 8.2 | 102 | 7.8 | |
| Rheumatic diseases | 7 | 5.9 | 88 | 7.4 | 95 | 7.3 | |
| Thyroid diseases | 8 | 6.7 | 121 | 10.2 | 129 | 9.9 | |
| Patient with a history of at least one related condition | 39 | 32.8 | 422 | 35.5 | 461 | 35.2 | |
| Patient's history | |||||||
| Chronic fatigue | 72 | 60.5 | 708 | 59.7 | 780 | 59.8 | NS |
| Chronic pain | 22 | 18.5 | 331 | 27.9 | 353 | 27.0 | .027* |
| Psychopathology prior to pain or fatigue | 2 | 1.7 | 26 | 2.2 | 28 | 2.2 | NS |
| Immune diseases | 1 | 0.8 | 6 | 0.5 | 7 | 0.5 | NS |
| Patient with a history of at least one related condition | 86 | 72.3 | 914 | 76.8 | 1.000 | 76.4 | NS |
| Apparent triggers | <.001* | ||||||
| Physical injury | 6 | 5.0 | 61 | 5.1 | 67 | 5.1 | |
| Poisoning | 1 | 0.8 | 5 | 0.4 | 6 | 0.5 | |
| Stressful life event | 21 | 17.6 | 260 | 21.8 | 281 | 21.5 | |
| Surgical intervention | 9 | 7.6 | 59 | 5.0 | 68 | 5.2 | |
| Infectious process | 32 | 26.9 | 155 | 13.0 | 187 | 14.3 | |
| Transfusion | 0 | 0 | 1 | 0.1 | 1 | 0.1 | |
| Pregnancy-childbirth | 0 | 0 | 134 | 11.3 | 134 | 10.2 | |
| Bariatric surgery | 0 | 0 | 1 | 0.1 | 1 | 0.1 | |
| Others | 23 | 19.3 | 187 | 15.7 | 210 | 16.0 | |
| None | 27 | 22.7 | 327 | 27.5 | 354 | 27.0 | |
NS: not significant; P: chi-squared test (comparisons by sex).
Chronic fatigue syndrome is a medical condition that is presently defined according to established diagnostic criteria. It mostly affects women, in a proportion that can be as high as 9 to 1, as was the case in our series, and as has been found in other immunoinflammatory processes (lupus erythematosus and multiple sclerosis).12,18 Perhaps because of its low prevalence among men, the literature includes few studies that assess the influence of gender in this syndrome. The study of Buchwald et al.,19 involving 348 patients with CFS (288 women and 60 men), demonstrated that lymph node involvement, pharyngitis, morning stiffness, intestinal dysfunction and FM were less common in men, who had higher physical functioning scores on the SF-36. This coincides with our findings, which show lower incidences of immunoinflammatory and neurovegetative symptoms, a lower prevalence of comorbidities (FM) and a better physical quality of life. The reports on gender influence in chronic fatigue are outnumbered by those referring to comorbidities associated with FM. Ruiz Pérez et al.20 demonstrated, in a cohort of 214 patients with FM (197 women and 17 men), that the time elapsed between the onset of symptoms and the diagnosis of the disease was longer in the men, who had a higher level of education and a higher rate of mental illness, and their disease had a greater impact on their families, when compared to the women. The authors attribute the higher prevalence of FM in women to hormonal, cultural and socioeconomic differences, as well as to the perception of the disease and the behavior and attitudes of women toward health care services. In a small group of 42 patients (21 women and 21 men), Miró et al.21 found that pain in the men was related to the quality of sleep and that the women had a lower pain threshold and a greater use of pain-killers, and were fearful of catastrophizing pain. In another report, Yunus et al.22 observed that the men with FM had fewer tender points, experienced less pain, fatigue and unrefreshing sleep, had a lower number of symptoms and were less likely to experience neurovegetative dysfunction in the form of irritable bowel syndrome. Over the last few decades, gender differences have been documented in several aspects of the disease, or diseases. Although these differences have been reported in rheumatic diseases and chronic pain syndromes, including those related to FM, the mechanisms implicated in the greater sensitivity to pain of women have not been fully understood. In this study, despite the fact that more of the women had histories of distress disorders, the HADS scale showed no difference between the sexes. The same can be said for the mental health and overall mental health dimension of the SF-36. This may indicate that the emotional symptoms do not produce the characteristics that differentiate between the sexes or that, at the emotional level, the disease affects men and women in the same way. We consider this point to be important, since both health professionals and the general public attribute a psychological or functional component to the development of CFS, when the most widely accepted hypothesis at the present time is that it is a neuroimmunological inflammatory process in patients with genetic susceptibility. This immune dysfunction would be triggered by a considerable number of factors (infections, physical and/or mental trauma, vaccines, toxins, anesthesia and/or childbirth, metals, chemicals) that cause the symptomatic complex of CFS, with intolerance to physical exercise as a peripheral aspect and severe cognitive dysfunction that includes impaired concentration and short-term memory as a central aspect. One of the major limitations of the study is the fact that, having a prospective cross-sectional design with enrollment at the time of diagnosis, it is not possible to assess the changes over time. Most of the patients were referred from primary care or specialized ambulatory care, and the waiting lists and lack of knowledge about the disease lead to a delay in the diagnosis. Thus, the time elapsed between the onset of symptoms and the diagnostic suspicion is longer than it should be. The study population was diagnosed in a unit specialized in CFS, meaning that the symptoms were more serious and the number of comorbidities greater than if recruitment had taken place in the primary care setting. This is the largest study on gender differences in CFS published to date.19 It shows that CFS was much less common among the men with respect to the women in the cohort studied. In terms of clinical phenotype, at the onset of CFS, male patients are younger, are unmarried and more often are skilled workers and, in many cases, the condition is triggered by an infectious process. Men have fewer muscle, immunological and neurovegetative symptoms. They also have fewer associated comorbidities (FN, musculoskeletal disorders, osteoporosis, anxiety/depression, thyroid disease) and their quality of life is better, preferentially in the components related to physical functioning and pain. There are studies that suggest that CFS is underdiagnosed in the general population, and that it is even more so among men. Future research will need to determine why men have a different clinical profile with regard to the symptoms, a circumstance that may involve some of the physiological differences (neuroendocrine system, cardiovascular system, musculoskeletal system, immune system, psychopathology and drug response).
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
AuthorshipThe authors of the manuscript have participated in the conception and design of the study, and in the collection, analysis and interpretation of the data, as well as in the writing, revision and approval of the submitted manuscript.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Faro M, Sàez-Francás N, Castro-Marrero J, Aliste L, Fernández de Sevilla T, Alegre J. Diferencias de género en pacientes con síndrome de fatiga crónica. Reumatol Clin. 2016;12:72–77.